In nature, the delicate construction of complex natural products is always dazzling. Simulating the biosynthesis pathway and orchestrating molecules with similar biological activities in the chemical laboratory has been challenging synthetic chemists for a long time. Biomimetic synthesis of natural products emerges as one of the most attractive field in the past decades.
Following the enzymatic cationic reactions in the past three years, the Professor Ran Hong’s group at Shanghai Institute of Organic Chemistry have embarked on the cation-initiated cyclization and designed an efficient protocol to afford functionalized benzazepine derivatives (Org. Lett.2009,11, 4036-4039). A novel ring-contraction reaction was also invented via a phenonium ion intermediate (Org. Lett.2010,12, 1696-1699). Based on the biosynthesis proposal of stemoamide, the Hong group developed a highly efficient biomimetic strategy and opened an avenue for more complex Stemona alkaloids in this family (Angew. Chem., Int. Ed.2011,50, 2787-2790). (See the group homepage:http://honglab.labways.com)
This work is generously supported by the National Science Foundation of China, the Ministry of Science and Technology of China, “CAS–100 Talents Program” and “Shanghai Rising Star Program”.
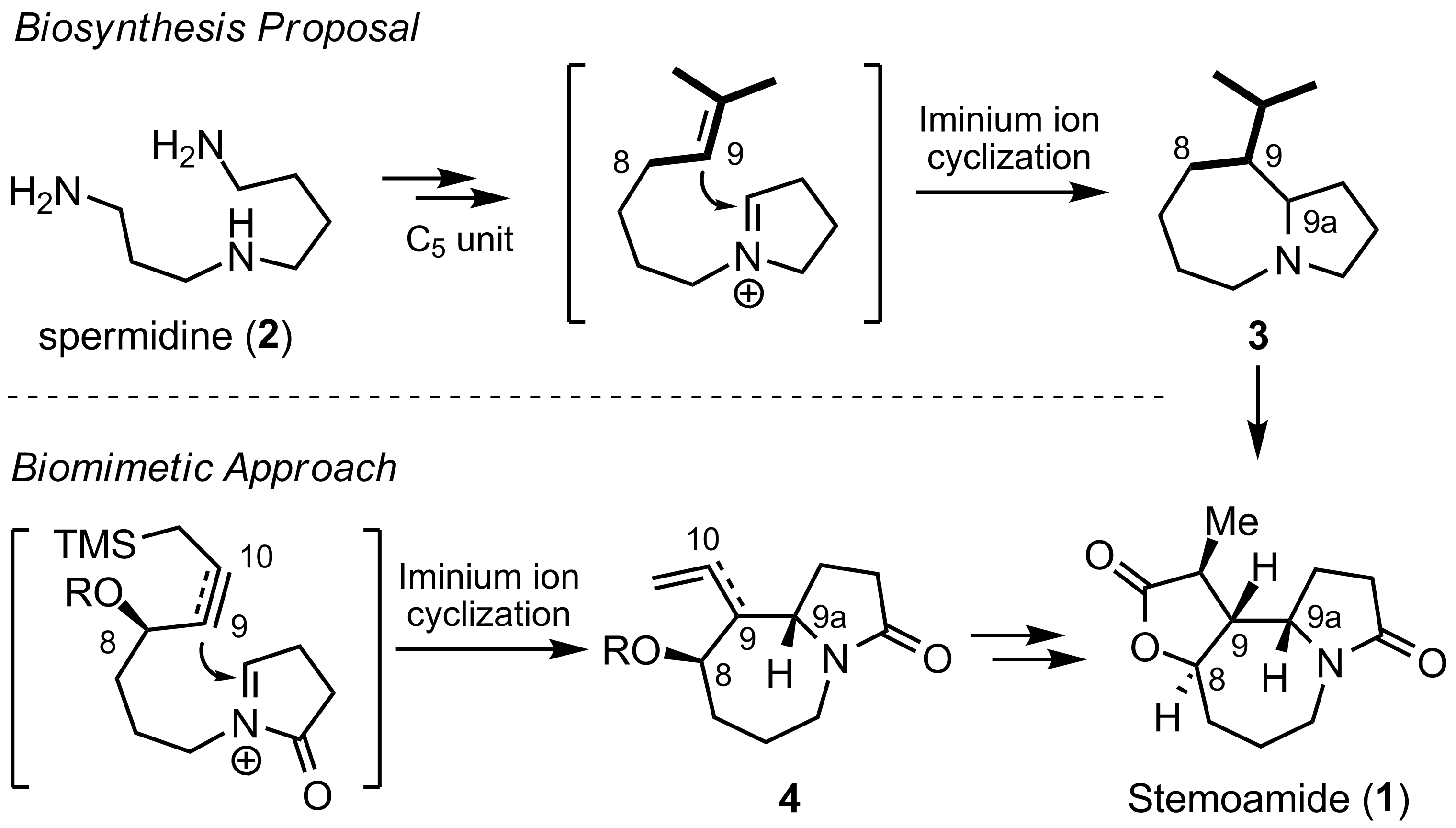
The highly efficient biomimetic strategy for stemoamide by Ran Hong’s group at SIOC.
(Image by Prof. Hong’s group@SIOC) |


