Trifluoromethylated organic compounds have been drawing great attentions due to their unique bioactivities. Trifluoromethyl groups in biologically active molecules played a key role in reinforcing their drug potency, which enabled them to withstand long exposure to the acidic conditions of the stomach, to cross into the blood stream, to be transported in sufficient quantity to the site of action, to perform the desired biological function efficiently and, finally, to be metabolized at an appropriate rate into non-toxic materials. However, the introduction of the trifluoromethyl group into common organic molecular frameworks under mild and selective conditions is one of the most challenging synthetic problems. On the other hand, trifluoromethyl)diarylsulfonium salts, which were first prepared by Yagupolskii in 1970s, are still limited to the electrophilic and radical reactions. No one knows their redox property until Ji-Chang Xiao reported his work.
Recently, Xiao and his group members investigated the reaction of sulfonium salts with metals in Key Laboratory of Organofluorine Chemistry at Shanghai Institute of Organic Chemistry, CAS. They found that S-(trifluoromethyl)diphenylsulfonium salts could be reduced by Fe, Pd(PPh3)4, Zn, Ag, CuI, Cu, etc. CuCF3, which was formed in the reaction of sulfonium salts with Cu, are very active trifluoromethylation intermediate. It can reactwith Ar1I to afford Ar1CF3. This reaction is suitable for a variety of iodo-substituted heteroaromatics. And the corresponding trifluoromethylated compounds were obtained in almost quantitive yield. This work has been published on Angew. Chem. Int. Ed.2011,50, 1896-1900, selected as hot paper, and highlighted by Synfacts (Synfacts,2011,5, 546). It is interesting that CuCF3 reacting with Ar2B(OH)2 in the presence of base also generated the corresponding trifluoromethylated products in good to excellent yield (Chem. Commun.2011,47, 9516-9518).
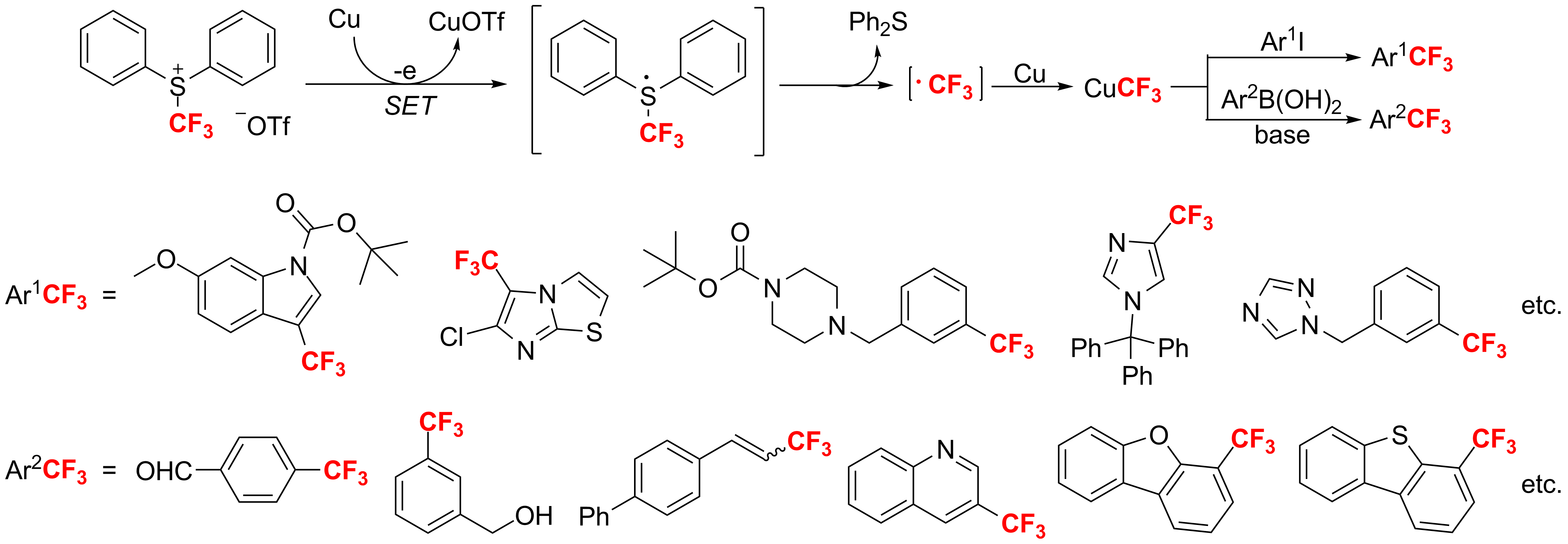
Trifluoromethylation of iodo-substituted aromatics and heteroaromatics by [Ph2SCF3]+[OTf ]- in the presence of copper. (Imaged by SIOC)
Furthermore, S-(trifluoromethyl)diphenylsulfonium salts can be reduced by inorganic reductants to form ·CF3, which is another discovery in xiao’s group. Trifluoromethyl radical (·CF3), which was generated in the reaction of [Ph2SCF3]+[OTf]- with rongalite or sodium hydrosulfite, can be captured by ArCH=CH2 and with air in this reaction, α-trifluoromethylated ketones were formed favorably (Chem. Commun.2011,47, 6632-6634).
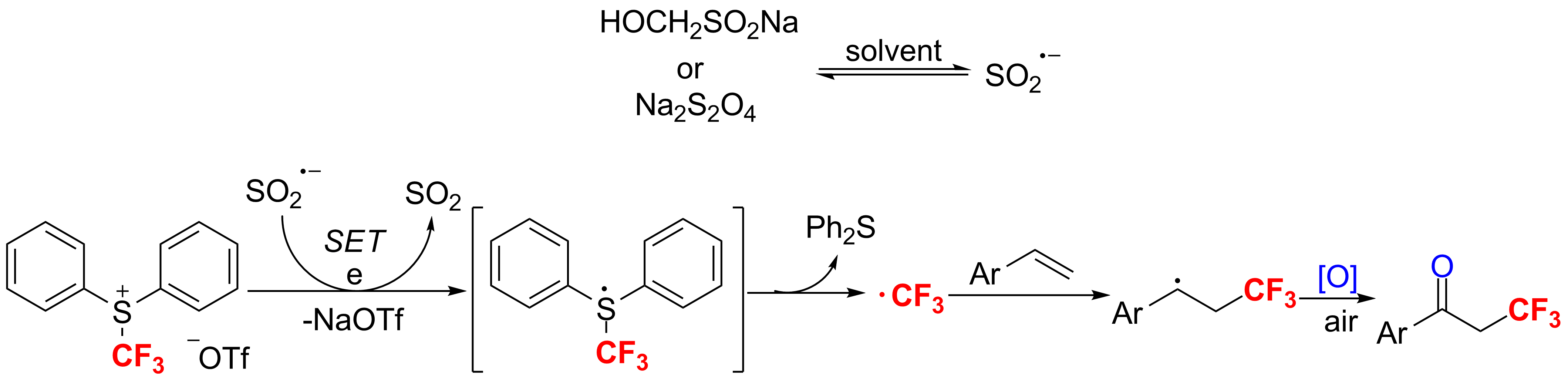
The oxidative-trifluoromethylation of styrenes.(Imaged by SIOC)
These research works were financially supported by the National Natural Science Foundation, Chinese Academy of Sciences.


