Over the last two decades, terminal imido complexes of early-transition-metals, which contain the M=N double bond, have attracted intensive interests and been extensively studied. The research on such complexes has revealed rich reactivities and applications in the group transfer and catalytic reactions. In contrast, the chemistry of rare-earth-metal terminal imido complexes remains unexplored. Owing to a relative mismatch in LUMO/HOMO orbital energies between the d0 rare-earth-metal ions and the imido groups, the RE=N (RE: rare-earth-metal) double bonds are highly polar and reactive. The rare-earth-metal terminal imido species once formed can easily assemble into more stable µ or µn (n = 3, 4) bridged bimetallic or multi-metallic species, or undergo reactions with solvents via C-H bond activation. Meanwhile, the chemistry of the rare-earth-metal terminal imido complexes is of great interest as the highly polar and reactive RE=N double bonds should lead to rich reactivities.
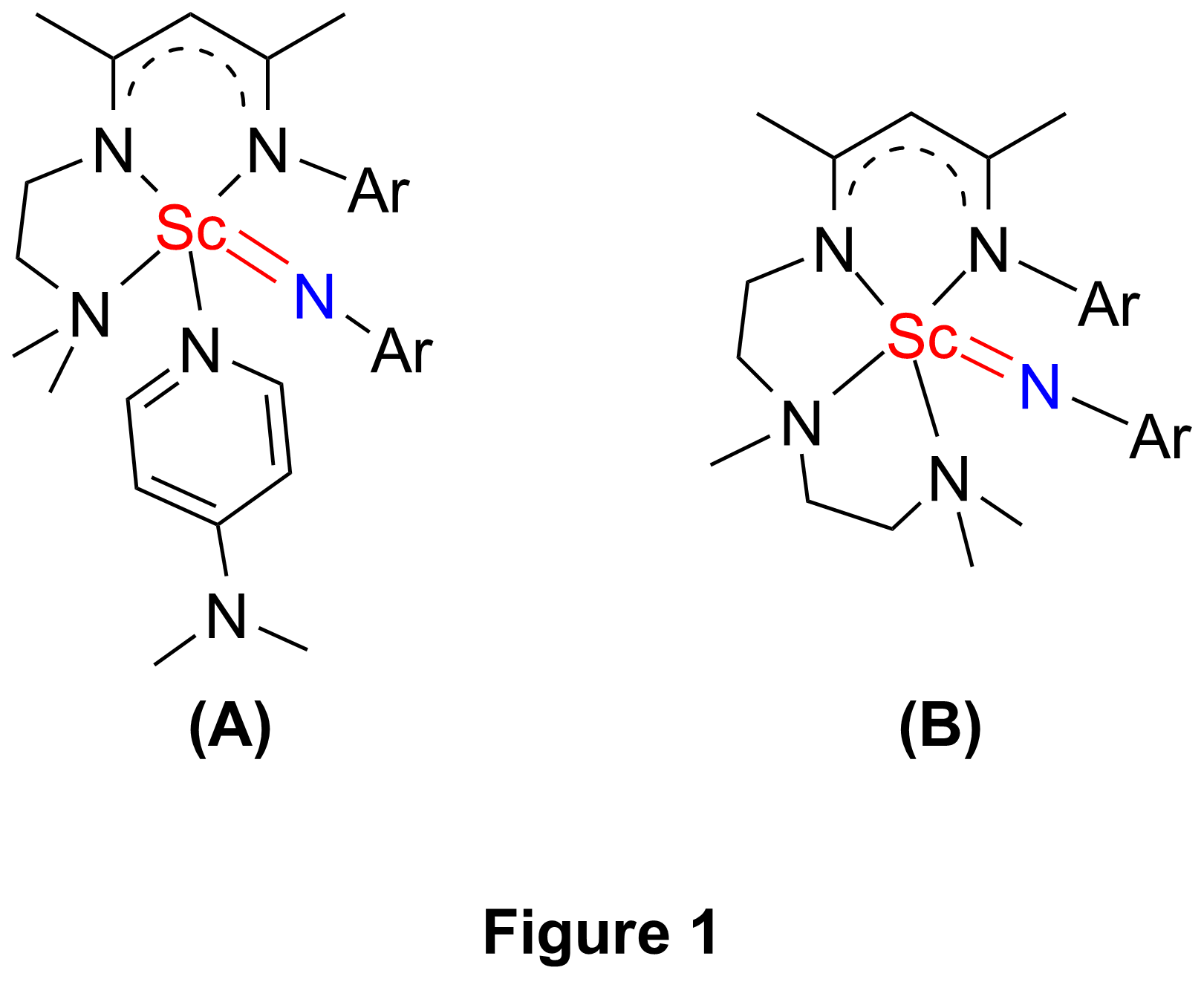
Recently, the research group of Yaofeng Chen of State Key Laboratory of Organometallic Chemistry at Shanghai Institute of Organic Chemistry had developed a type of b-diketiminato based tridentate ligands on considering both steric and electronic features of the ligand, and these ligands exhibited excellent effect on stabilizing highly reactive rare-earth-metal dialkyl complexes (Organometallics 2008, 27, 758). By using of one of these ligands, Chen’s group synthesized the first example of rare-earth-metal terminal imido complex, a scandium terminal imido complex (Chart 1(A)), from the corresponding anilido methyl complex in the presence of external Lewis base DMAP. The complex was structurally characterized by single-crystal X-ray diffraction, and DFT studies on the complex were also performed(Chem. Commun., 2010, 46, 4469). Then they designed and synthesized a b-diketiminato based tetradentate ligand. With this ligand, they were able to prepare a scandium terminal imido complex (Chart 1(B)) in the absence of external Lewis base DMAP.
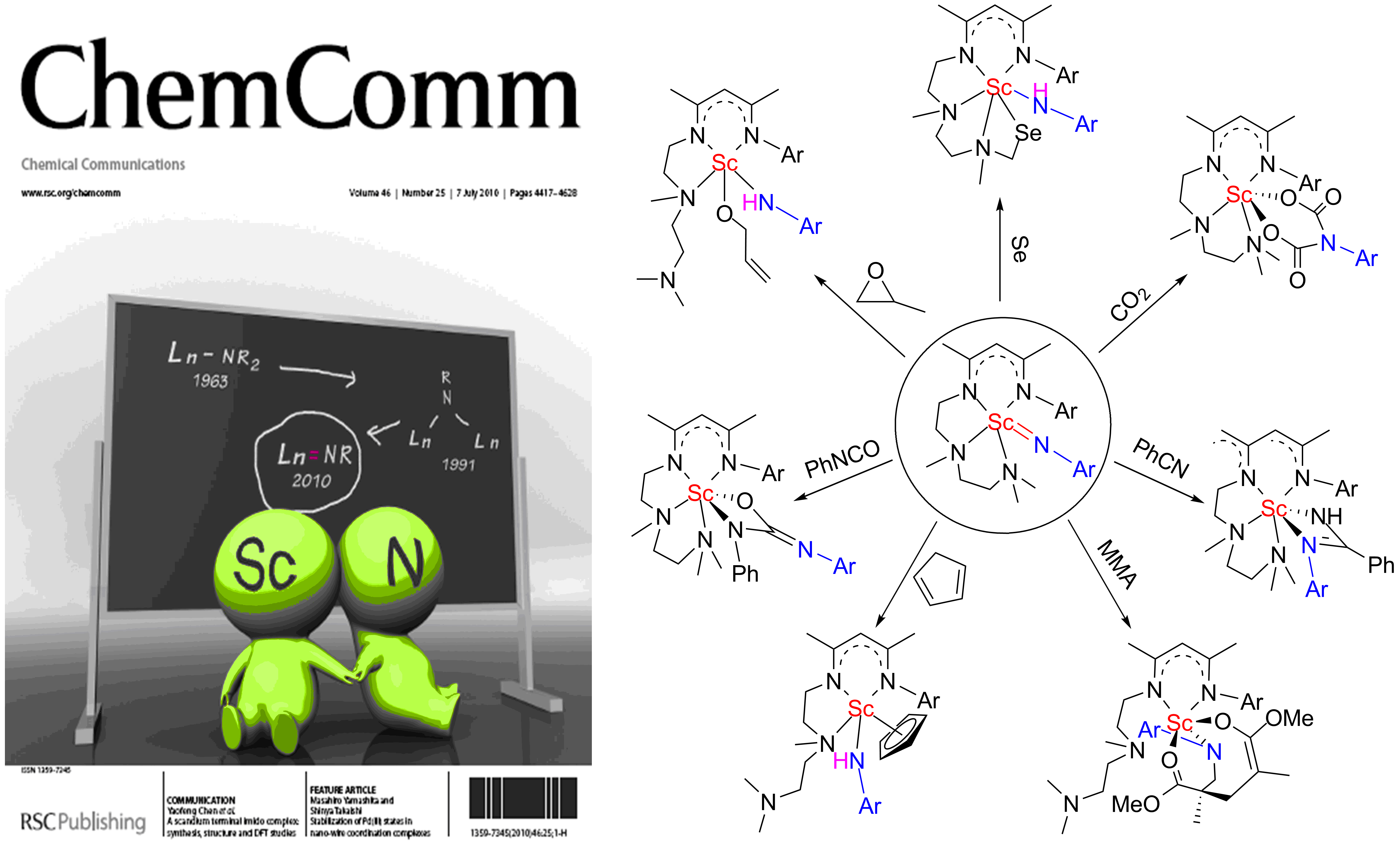
The brief reactivity study on the scandium terminal imido complexes showed that these complexes are capable for the activation of elemental selenium(Chem. Commun., 2011, 47, 743), and undergo cycloaddition with CO2 and a series of unsaturated organic small molecules(Angew. Chem. Int. Ed. 2011, 59, 7677). These results demonstrated that the rare-earth-metal terminal imido complex can be synthesized and stabilized by using the suitable ancillary ligand, hinting a light on the synthesis of rare-earth-metal complexes containing RE=C(or P) double bond. The reactivity study on the scandium terminal imido complexes also showed some interesting reactivity from the Sc=N double bond.
 |


