Natural products, have always been one of the major sources of anticancer drugs. Biosynthetic studies of biologically natural products with fascinating molecular architecture focus on characterizing the biological and chemical process of naturally “total synthesis” on the basis of gene cluster, pathway and enzymatic reaction. Prof. Gong-Li Tang’s group in State Key Lab. of Bioorganic and Natural Products Chemistry of SIOC has been engaged in the biosynthetic studies of antitumor natural products for many years. Recently, they made significant progress in two projects.
Yatakemycin (YTM), an antitumor natural product, represents the most potent member of a class of potent anticancer natural products including CC-1065 and duocarmycins. The biosynthetic gene cluster of YTM was identified by genome scanning, it consists of thirty-one open reading frames (ORFs) and was localized to a 36 kb DNA segment. Inactivation of ytkT, which encodes a radical S-adenosylmethionine (SAM) protein, created a mutant strain that failed to produce YTM but accumulated a new metabolite, which was structurally elucidated as a precursor that was related to the formation of the cyclopropane ring. Biochemical characterization of the radical SAM-dependent enzyme YtkT revealed that it is a novel C-methyltransferase and contributes to an advanced intermediate during formation of the cyclopropane ring through a radical mechanism in the YTM biosynthetic pathway. On the basis of in silico analysis, genetic experiments, structure elucidation of the novel intermediate, and biochemical characterization, a biosynthetic pathway for yatakemycin was proposed, which sets the stage to further investigate the novel enzymatic mechanisms and engineer the biosynthetic machinery for the production of novel analogs.
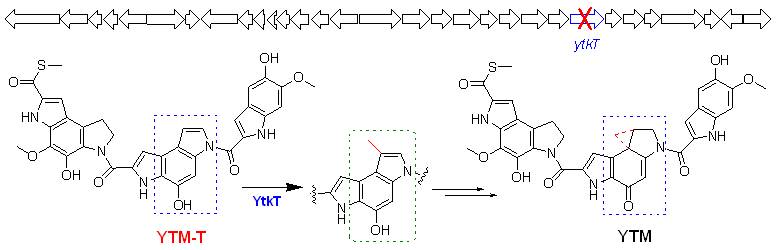
Fig. 1 Characterization of YTM gene cluster revealing a radical S-adenosylmethionine dependent methyltransferase and highlighting spirocyclopropane biosynthesis. (imaged by Tang G.L.@SIOC)
Nonribosomal peptide synthetases (NRPSs) usually catalyze the biosynthesis of peptide natural products by sequential selection, activation, and condensation of amino acid precursors. It was reported that some fatty acids, α-ketoacids or α-hydroxyacids originated from amino acid metabolism, and polyketide derived units, can also be utilized by NRPS assembly lines as an alternative to amino acids.Ecteinascidin 743 (ET-743), naphthyridinomycin (NDM) and quinocarcin (QNC) are three important antitumor natural products belonging to the tetrahydroisoquinoline family. Although ET-743 has been approved as an anticancer drug, the origin of an identical two-carbon (C2) fragment among these three antibiotics has not been elucidated despite much effort on the biosynthetic research in the past 30 years. Two unexpected two-component transketolases (TKases), NapB/NapD in NDM biosynthetic pathway and QncN/QncL in QNC biosynthesis, were elucidated to catalyze the transfer of a glycolaldehyde unit from ketose to the lipoyl group to yield the glycolicacyl lipoic acid intermediate, then transfer the C2 unit to an acyl carrier protein (ACP) to form glycolicacyl-S-ACP as an extender unit for NRPS. These results demonstrate a novel NRPS extender unit directly derivated from ketose phosphates through α,β-dihydroxyethyl-ThDP and lipoyl group tethered ester intermediate catalyzed by the new TKase-ACP platform in the context of NDM and QNC biosynthesis, which also highlight the biosynthesis of ET-743. This novel hybrid system and precursor are distinct from the previously described universal modes involving the NRPS machinery. It exemplifies a new strategy in hybrid NRPS biochemistry and enriches the diversity of precursors for NRPS combinatorial biosynthesis. (Proc. Natl. Acad. Sci. USA,2012,109, 8540-8545)
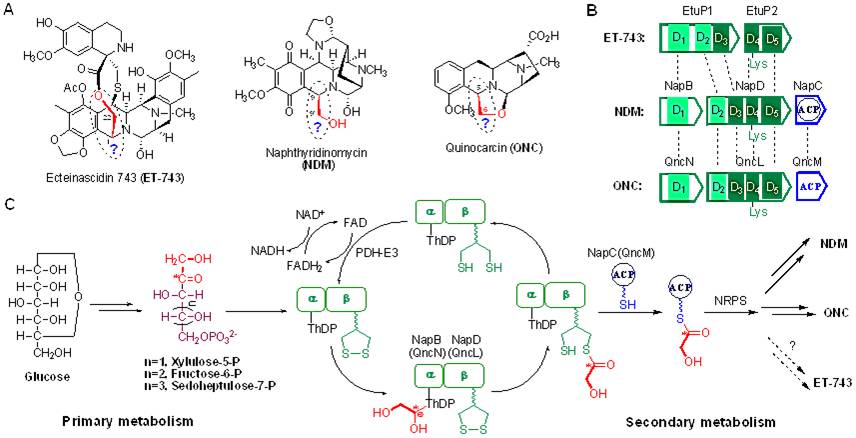
Fig. 2 Hijacking a hydroxyethyl unit from a central metabolic ketose into a nonribosomal peptide assembly line involved in the biosynthesis of tetrahydroisoquinoline natural products. (imaged by Tang G.L.@SIOC)


