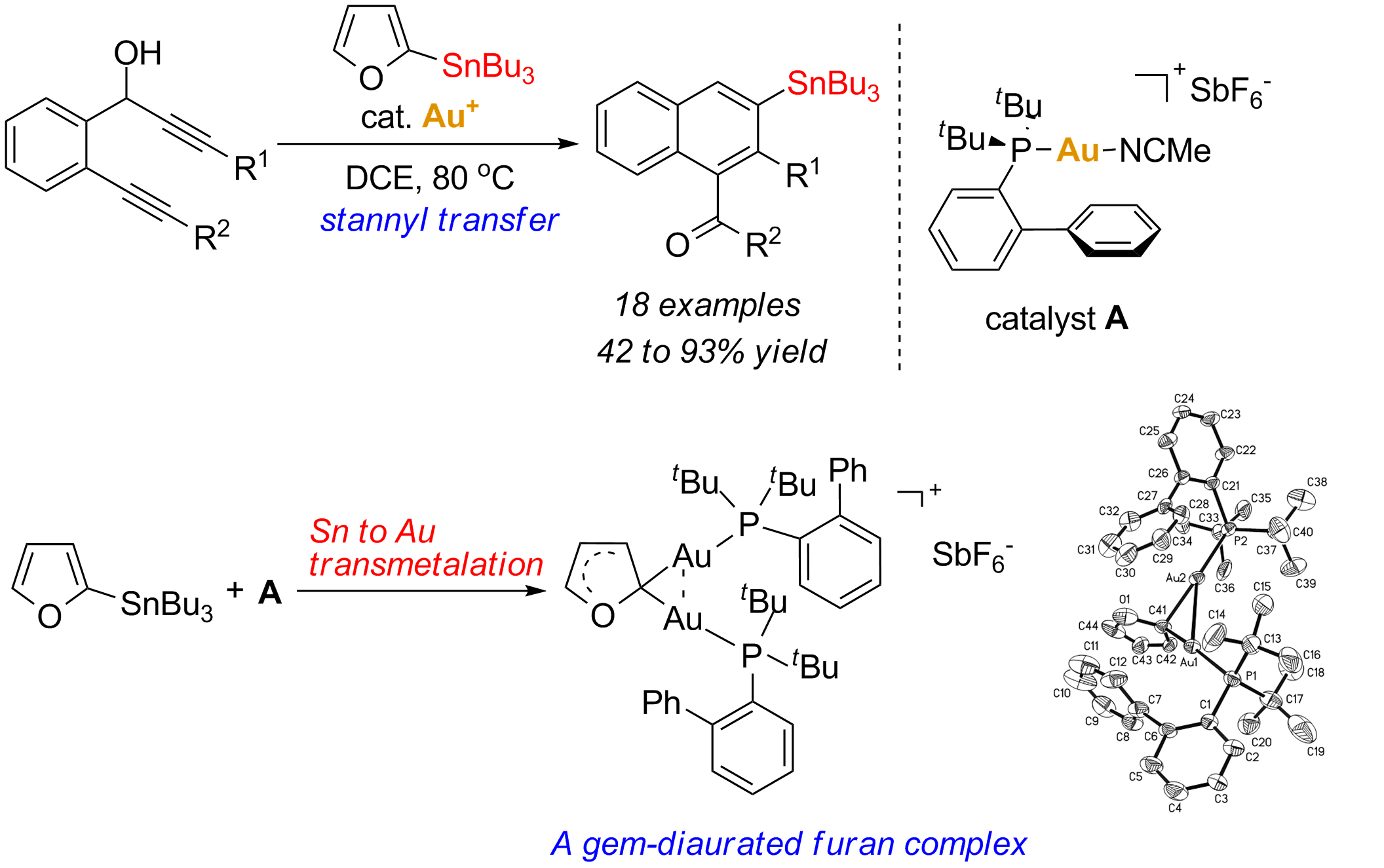
In recent years, gold complexes and salts have emerged as powerful homogeneous catalysts for a wide variety of synthetic transformations due to their superior chemoselectivities and activaties. Especially, gold catalysts can act as efficient alkynophilic Lewis acids to activate the p-systems towards the nucleophilic attack. Despite the significant diversity of these reactions, the functionalization of the organogold intermediates featured with a gold-carbon s bond still remains as a major challenge in this area. Gold species are captured primarily by a proton, more seldom with alternative electrophiles such as carbocation, sulfonyl, silicon and halogen electrophiles. Organogold complexes have been proven to be good precursors for transmetalation reactions toward Ni, Pd,Rh, Sn, Fe, Ru,etc. However, most of the studiesinvolved the use of a pre-prepared stoichiometric amount of organogold reagent, whereas a catalytic version involving transformation of a gold intermediate generated in situ by a gold-catalyzed reaction is quite rare. Recently, researchers from Shanghai Institute of Organic Chemistry have disclosed the first example of a single gold catalyzed stannyl transfer reaction of 1,6-diyne-4-en-3-ols with 2-tributylstannylfuran. This transformation provides an attractive new route for diverse substituted naphthylstannanes in a regioselective manner, which, coupled with Pd-catalyzed Stille cross-coupling reactions, allowed for readily access to highly functionalized naphthalenes. Mechanistic study revealed that Au/Sn transmetalation and gold-catalyzed destannylation might be involved in the domino process. They also showed the first direct crystallography evidence for Sn-to-Au transmetalation using cationic gold complex. The method may find further application for the functionalization of the organogold intermediates proposed in various gold-catalyzed reactions (Chen, Y.; Chen, M.; Liu, Y. Angew. Chem. Int. Ed. 2012, 51, 6181).
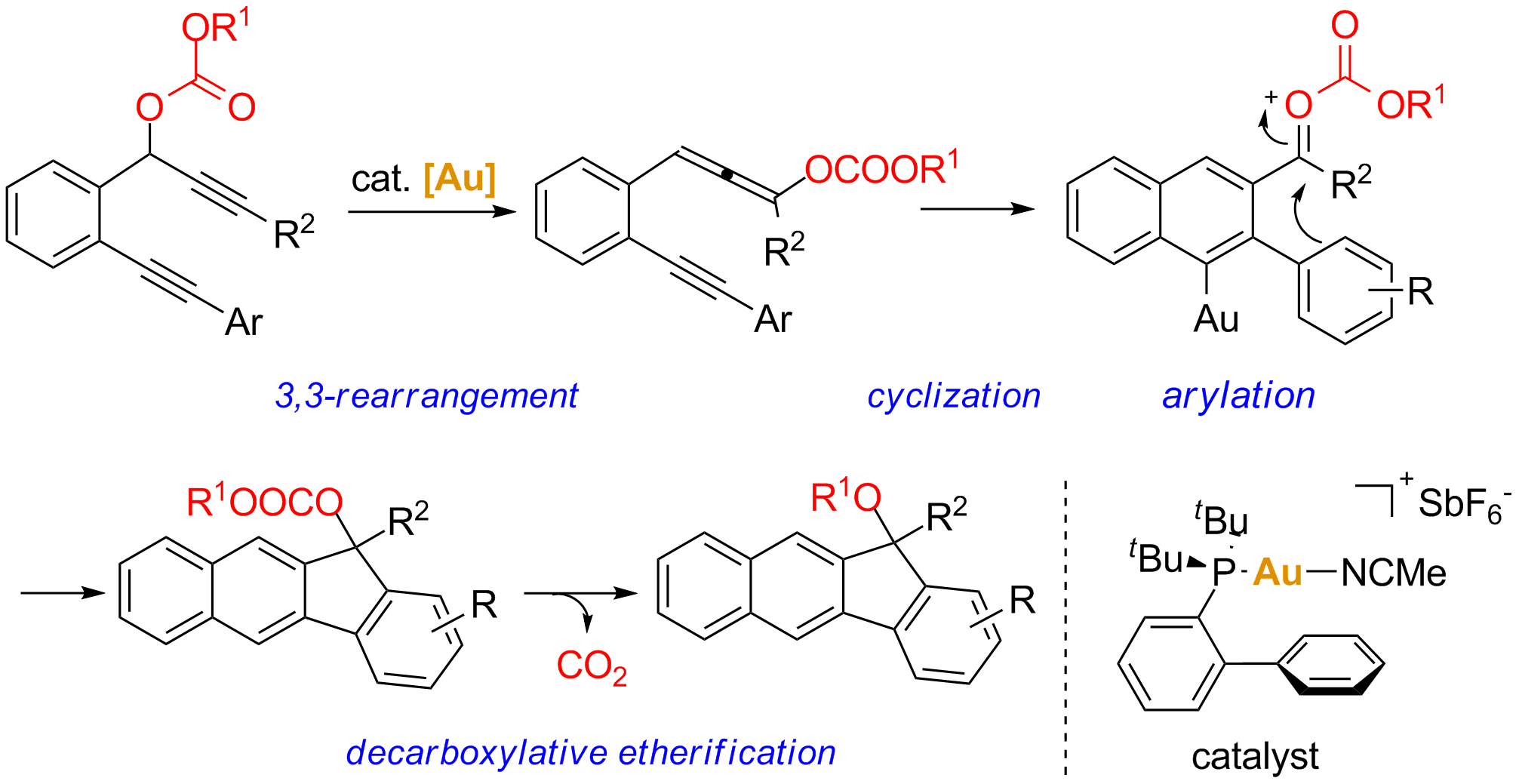
They also developed a new cascade reaction of 1,6-diynyl carbonates to highly substituted benzo[b]furans involving rare reaction patterns of arylation of oxocarbenium ion intermediates and decarboxylative etherification. They suggest that when propargyl carbonates are employed instead of carboxylates, it is possible to form more stable oxocarbenium ion intermediates, which can be further attacked by nucleophiles. Experimental results indicate that the in situ-formed oxocarbenium ion intermediates, derived from gold-catalyzed 3,3-rearrangement and 6-endo-dig cyclization, undergo intramolecular arylation and subsequent decarboxylative etherification to furnish the final ether products. The results also indicate that the substituents on the migrating groups may play an important role for defining the reaction pathway in gold catalyzed rearrangement reactions of propargyl esters (Chen, Y.; Chen, M.; Liu, Y. Angew. Chem. Int. Ed. 2012,51, 6493).
This project was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology (973 Program), and the Chinese Academy of Sciences. |


