Decarboxylative halogenation of carboxylic acids, the Hunsdiecker reaction, is one of the fundamental functional group transformations in organic chemistry. Hunsdiecker reactions have long attracted organic chemists’ attention because of the easy availability and high stability of carboxylic acids and the important value of organic halides in organic synthesis. The initial method used strictly anhydrous silver carboxylates to react with bromine. Because of the difficult preparation of dry silver carboxylates, several methods have thus been developed to simplify the procedure. However, these methods suffer from their own limitations, which limit their application in organic synthesis.
After over three years of research, the Chaozhong Li group in the Key Laboratory of Synthetic Chemistry of Natural Substances, Shanghai Institute of Organic Chemistry (SIOC), developed the first examples of transition metal-catalyzed Hunsdiecker reactions of aliphatic carboxylic acids (J. Am. Chem. Soc. 2012, 134, 4258–4263). Thus, with Ag(Phen)2OTf as the catalyst, aliphatic carboxylic acids underwent efficient decarboxylative chlorination by treatment with tert-butyl hypochlorite under mild conditions (Eq 1). This method enjoys not only the wide substrate scope and functional group compatibility but also an excellent chemoselectivity. The reactivity of carboxylic acids increase in the order of aromatic << 1o alkyl < 2o alkyl < 3o alkyl ≈ benzylic. The mechanistic investigations indicated that the reactions proceed via a Ag(II)-mediated radical process.
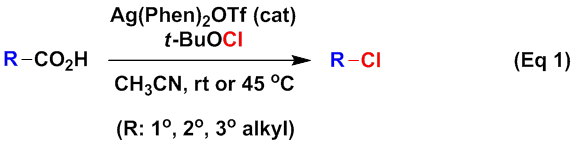
Taking advantage of the unique property of Ag(II) ions in oxidative decarboxylation, the Li group further introduced the radical decarboxylative alkynylation of aliphatic carboxylic acids (J. Am. Chem. Soc. 2012, 134, 14330–14333). As shown in Eq 2, with AgNO3 as the catalyst and K2S2O8 as the oxidant, the reactions of aliphatic acids with ethynylbenziodoxolones led to the corresponding decarboxylative alkynylation products. The reactions proceeded in aqueous solution under mild conditions with a broad substrate scope and functional group compatibility, demonstrating the advantage of radical processes in C(sp3)–C(sp) bond formations. In addition, the byproduct o-iodobenzoic acid serves as the starting material for ethynylbenziodoxolones and thus can be recycled in theory.
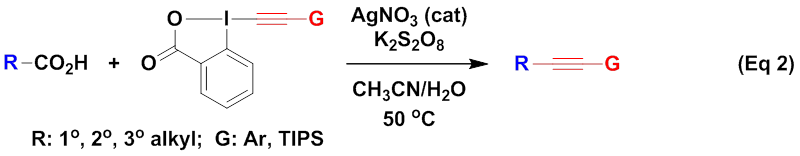
On the basis of the above works, the Li group focused on the decarboxylative fluorination of aliphatic acids. Their investigation revealed that (Eq 3), with AgNO3 as the catalyst and the commercially available Selectfluor (1-Chloromethyl-4-fluorodiazoniabicyclo[2,2,2]octane bis(tetrafluoroborate)) as the fluorine source, aliphatic acids underwent decarboxylative fluorination in aqueous solution at room temperature to 65 oC, leading to the efficient synthesis of the corresponding alkyl fluorides (J. Am. Chem. Soc. 2012, 134, 10401–10404). The mild conditions and simply operations make this method highly practical.
Similar to the above-mentioned chlorination, the fluorination reactions also enjoy a wide scope of application, excellent functional group compatibility and chemoselectivity. However, the difference lies in that the fluorination can not take place based on Ag(II)-mediated radical processes. In the meantime, the mechanistic study also indicated that the direct interaction between alkyl radicals and Selectfluor follows a single electron transfer mechanism, and therefore is unlikely to produce alkyl fluorides efficiently in aqueous media. The Li group then proposed the following novel mechanism as illustrated in Figure 1. The oxidation of Ag(I) by Selectfluor generates the Ag(III)–F intermediate presumably via oxidative insertion. The trivalent silver species then undergoes single electron transfer with a carboxylate anion to give the divalent silver intermediate Ag(II)–F and a carboxyl radical. The fast decarboxylation of the carboxyl radical provides the corresponding alkyl radical, which then abstracts the fluorine atom of the adjacent Ag(II)–F to afford the product alkyl fluoride and regenerates the Ag(I) catalyst. This mechanism brings forward the concept “metal-assisted fluorine-atom-transfer”, which provides a new way of thinking and research method for C(sp3)–F bond formations. It is expected that the silver-catalyzed radical fluorination reactions will play an important role in the synthesis of fluorinated organic molecules as well as fluorinated materials. The research in this aspect is currently underway.
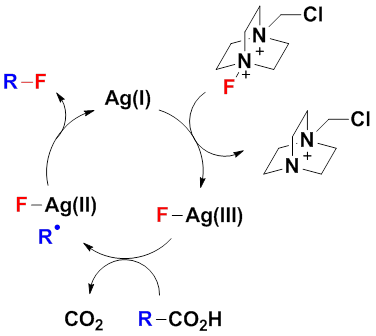
Fig. 1 The novel mechanism proposed by Li group.(imaged by Chaozhong Li@SIOC)
This project was supported by the National Natural Science Foundation of China and by the National Basic Research Program of China (973 Program).


