Low molecular weight heparins (LMWHs) have been used as anticoagulant agents for over two decades. Enoxaparin sodium, one of the LMWHs related brand-name drug, represents top 20 best sale drugs in the global pharmaceutical market. On July 23, 2010, FDA approved enoxaparin sodium as the generic drug for anticoagulant therapy. Thus, demonstrating “sameness” for enoxaparin sodium requires “state-of-the-art” analytical methods to show that the structural features of oligosaccharides in the generic enoxaparin sodium are equivalent to those in the original brand-name drug. Moreover, potential pharmaceutical applications of LMWHs for other diseases such as cancer, diabetes, and senile dementia have also been reported. However, their structures have not been fully characterized and the structure-function relationship has not been fully understood due to their high structural complexity. LWMHs are derived from unfractionated heparin (UFH) by enzymatic or chemical depolymerization, therefore, they inherit the repeating disaccharide units composed of a glucosamine residue and an uronic acid residue (iduronic or glucuronic acid). The various sulfation patterns, acetylation modifications, epimerization of iduronic acid to glucoronic acid, and various chain lengths lead to considerable heterogeneity. Compared to UFH, LMWHs are more complicated in composition and sequence. These alterations give rise to the different biochemical and pharmacological properties observed in various LMWHs.
A new strategy for the comprehensive structural analysis of LMWHs has been proposed by the group of Prof. Kang Jingwu at the SIOC. It based on the combination of ultra-performance size exclusion chromatography/electrospray quadruple time-of-flight mass spectrometry (UPSEC/Q-TOF-MS) for “top-down” profiling analysis and capillary zone electrophoresis (CZE) for “bottom-up” quantitative analysis of the components of LMWHs. More than 70 components, including oligosaccharides with special structures such as 1,6-anhydro rings, saturated uronic acid at the non-reducing end, and odd number saccharides units were identified with UPSEC/Q-TOF-MS. Further, a more detailed compositional analysis was accomplished by CZE analysis. PEG10000 and MgCl2 were added to background electrolyte to separate those saccharides with the nearly same charge-to-mass ratio. Baseline separation and quantification of all the building blocks of the most complex LMWH enoxaparin—including ten disaccharides, one trisaccharide, two tetrasaccharides, and, of particular importance, four 1,6-anhyro derivatives, was achieved using CZE for the first time. The peaks of oligosaccharides without commercially available standards were assigned on the basis of the linear correlation between the electrophoretic mobilities of oligosaccharides and their charge-to-mass ratios. These two approaches are simple and robust for structural analysis of LMWHs.
Compared with the SAX-HPLC method, the new CZE method is simpler, faster, and more cost-effective. The approach can be the key technique for quality control of LMWHs related drugs and for elucidating the contribution of each structural feature to the bioactivity of LMWHs. This work has been published in Analytical Chemistry,2013,85, 1819–1827 on February 5th 2013.
This work was financially supported by the funding from the National Natural Science Foundations of China, the Ministry of Science and Technology of China and Chinese Academy of Sciences.
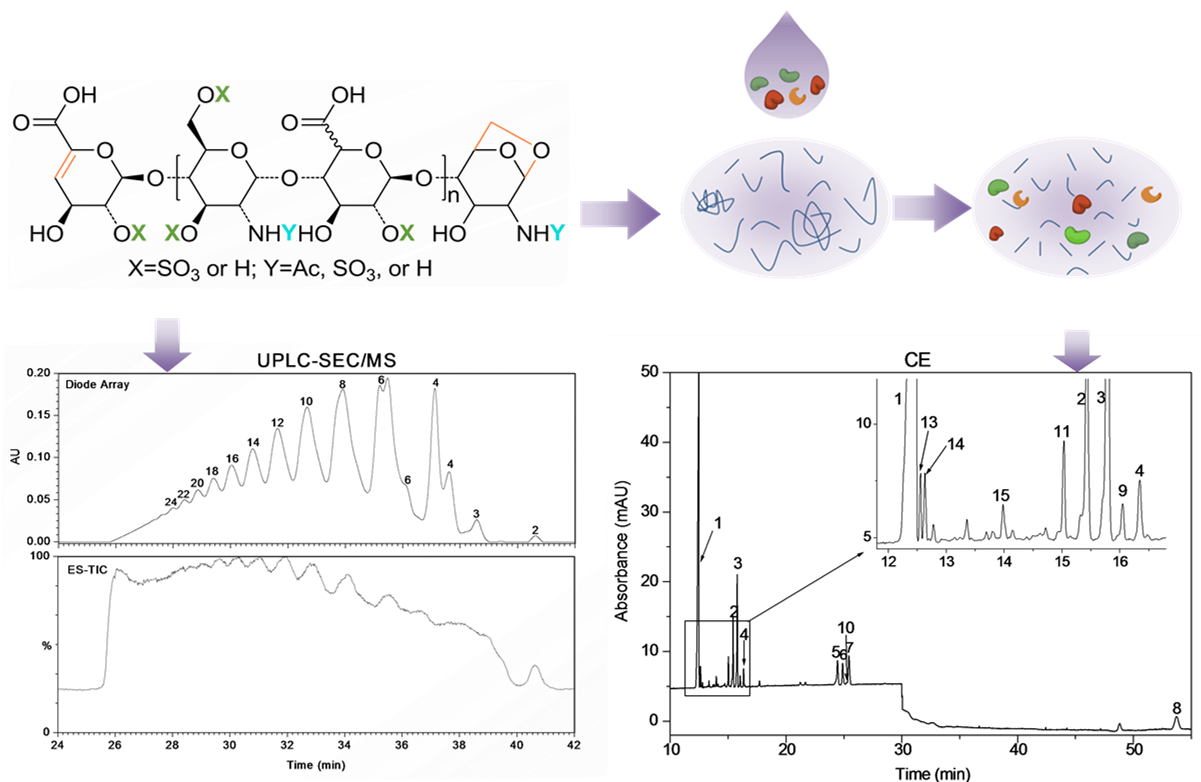
The process of the the approach which can be the key technique for quality control of LMWHs related drugs. (imaged by KANG JIngwu@ SIOC)


