Organic transistors have great potential applications in flexible display, RFID card and sensors because they can be fabricated in larger scale on flexible substrates at low cost. High performance organic semiconductors are the key part of organic transistors and determine the performance of organic transistors. Recently, Professor Hongxiang Li’s research group at Shanghai Institute of Organic Chemistry, CAS, make new progress in high performance organic semiconductors. They designed and synthesized a new type of p – channel organic semiconductor, 6,13-dichloropentacene (DCP). A facile method to synthesize gram scale of DCP was developed. The single crystal transistors of DCP displayed high hole mobility with the maximum mobility up 9.0 cm2/Vs. Unlike most of the organic semiconductors, DCP molecules have a strong tendency to adopt a slipped π-π stacking mode regardless of the changes of substrates (OTS-modified or bare SiO2/Si substrate) and deposition conditions.The π-π stacking with large intermolecular overlap is responsible for the high mobility of DCP ribbons. These properties, combined with the high stability and easy synthesis, demonstrate the potential application of DCP inorganic electronics ( Adv. Mater. 2013,15, 2229-2233).
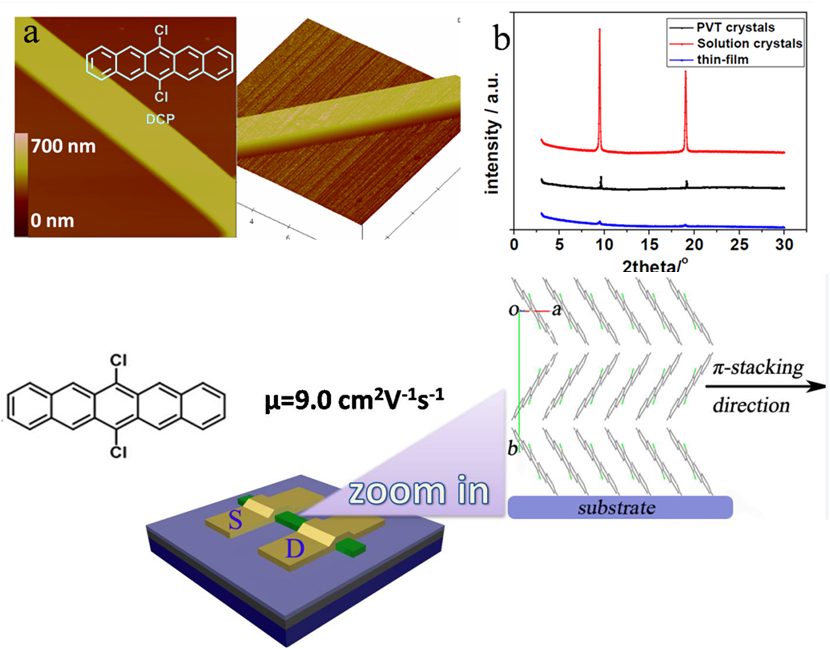 Single and large-area aligned crystalline microribbons of 6,13-dichloropentacene (DCP) (Imaged by Hongxiang Li@SIOC)
Besides p – channel organic semiconductors, they also make progress in n- channel organic semiconductors. Base on their previous research results on thiophene quinoidal organic semiconductors which is solution processable and exhibits high performance under ambient conditions ( Chem. Mater. 2011, 23, 3138-3140), they increased the conjugation length of thiophene quinoidal compounds and synthesized series of dicyanomethylene-substituted 2,5-di(thiophen-2-yl)thieno[3,2-b]thienoquinoids. In these compounds, the soluble alkyl chains (2-decyltetradecyls) were substituted at the different positions of the conjugate backbone, which is aimed to investigate the effect of alkyl chain orientations to device performance. All compounds exhibited ambient stable TFT performance, however their performances were dramatically changed. The widely variation of TFT performances were ascribed to the different molecular packing patterns in thin film and different film morphology of these compounds which was caused by the alkyl chain orientations. These results indicated that the change of side alkyl chain orientations is an important method and should be paid more attentions to rational design of high performance OSCs ( Adv. Funct. Mater.2013,23, 2277-2284).
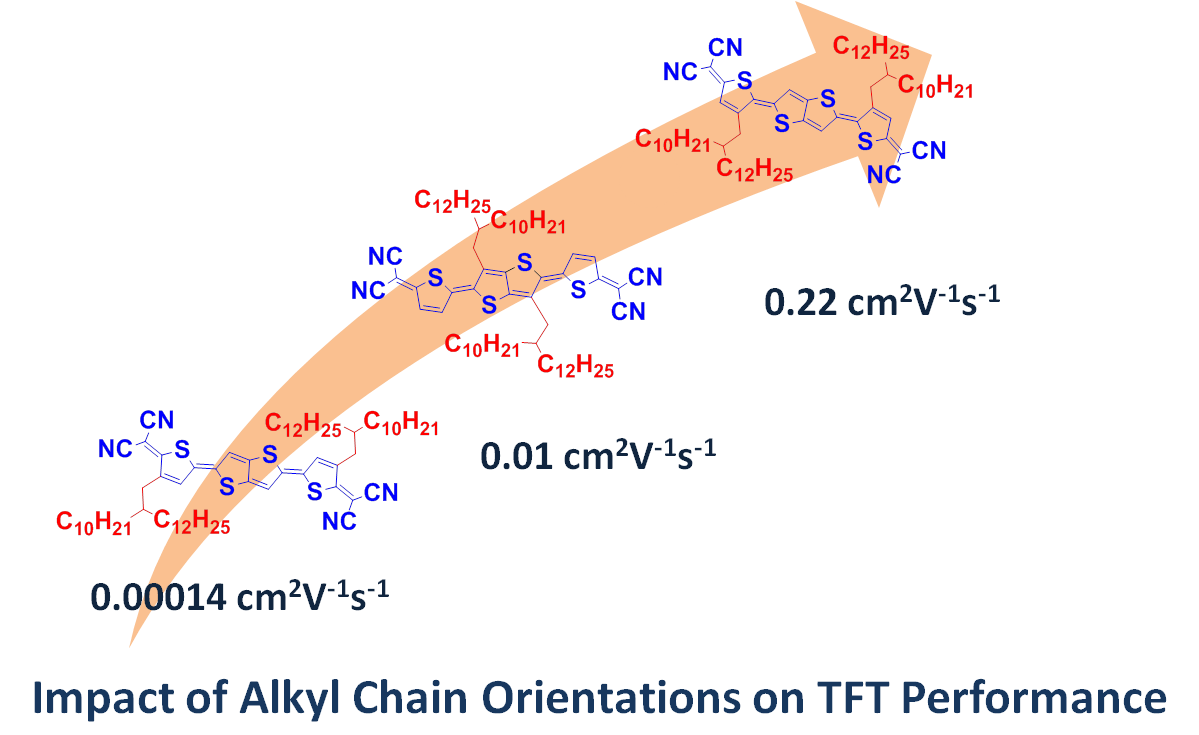 (Imaged by Hongxiang Li@SIOC)
This work was supported by National Natural Sciences Foundation of China and National Basic Research Program of China.
|


