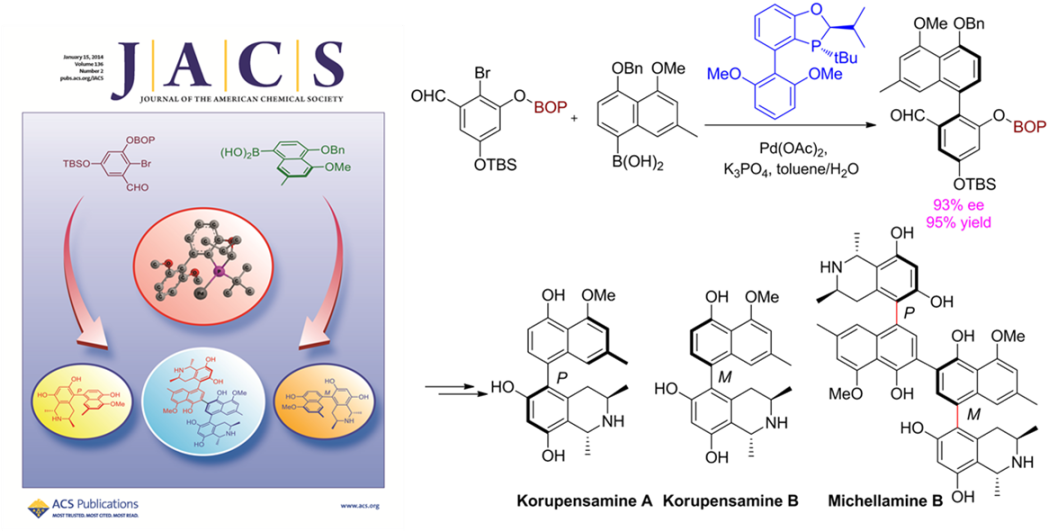
There exist numerous chiral biaryl natural products in nature with interesting biological functions. For example, both Korupensamine A and its atropisomer Korupensamine B, isolated from Cameroon Liana Ancistrocladus korupensis, provide strong antimalarial activities. Their heterodimer Michellamine B is a strong anti-HIV-1 and anti-HIV-2 agent, once used for clinical trial. Construction of chiral biaryl natural products by efficient asymmetric Suzuki-Miyaura coupling reactions under mild reactive conditions is among most attractive, yet remains one of most challenging areas in organic synthesis.
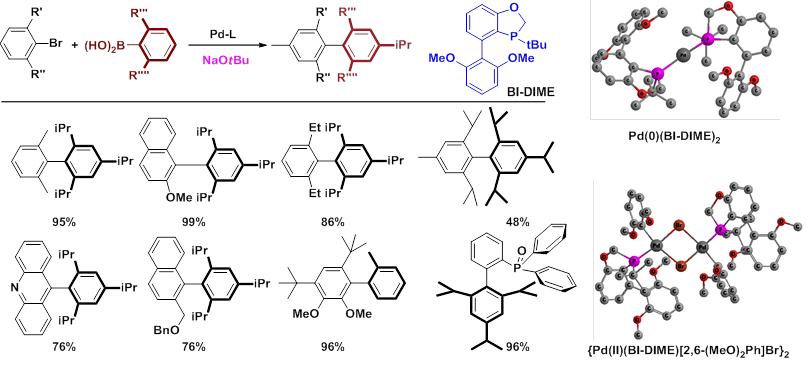
The development of a highly reactive, stereoselective, and practical methodology of asymmetric Suzuki-Miyaura coupling has thus become a major research goal in Professor Wenjun Tang’ research group at State Key Laboratory of Bioorganic & Natural Products Chemistry of Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences. A novel solution to this problem was proposed by them with the development of a catalyst composed of a chiral bulky monophosphorus ligand as well as the utilization of a 2nd interaction between two coupling partners (Synlett,2013,24, 2465). Toward this end, the coupling catalyst must be efficient for sterically demanding Suzuki-Miyaura coupling. The group have thus designed and developed a series of rigid and sterically bulky monophosphorus ligands for extremely hindered aryl-aryl and aryl-alkyl Suzuki-Miyaura couplings, which have significantly expanded the synthetic utilities of cross-coupling reactions (Chem. Eur. J.2013,19, 2261;Angew. Chem., Int. Ed.2010,49, 5879; Org. Chem. Front.2014, 225.).
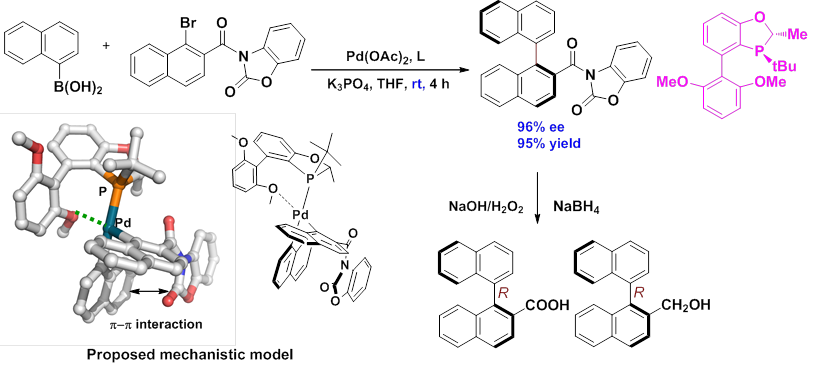
Meanwhile, a practical and highly stereoselective asymmetric Suzuki-Miyaura coupling methodolgy for functionalized biaryls was developed with a P-chiral monophosphorus ligand developed in their group, and the influence of 2nd interaction between two coupling partners on enantioselectivity was explored and studied (Org. Lett.2012,14, 2258).
Recently, a more practical and efficient asymmetric Suzuki-Miyaura coupling was developed with excellent enatioselectivities and functional group compatibility, taking advantage of a polar 2nd interaction between coupling partners (J. Am. Chem. Soc.2014,136, 570-573) . The methodology has been successfully applied for the first time in natural product syntheses and allowed the syntheses of both korupensamine A, B, and Michellamine B through catalytic asymmetric Suzuki-Miyaura coupling. This strategy should greatly facilitate the concise syntheses of a wide range of chiral biaryl natural products. The project is financially supported by the “Thousand Plan” Youth program, the National Natural Science Foundations of China, Science and Technology Commission of Shanghai Municipality and Chinese Academy of Sciences.