|
|
| Significant Progress Was Made in the Biosynthetic Studies of Antitumor Natural Product FR901464 by SIOC (II) |
 |
Text Size: A A A |
|
FR901464, an antitumor natural product, representing a new class of potent anticancer small molecules targeting spliceosome and inhibiting both splicing and nuclear retention of pre-mRNA, have drawn considerable interests for chemical synthesis, pharmaceutical development, and chemical biology. The researchers of State Key Laboratory of Bio-organic and Natural Product Chemistry at Shanghai Institute of Organic Chemistry, CAS, reported the biosynthetic gene cluster of FR901464, which revealed a complex polyketide synthase (PKS) system with several novel features (J. Am.Chem.Soc.2011,133, 2452-2462). Subsequently, an unprecedented domain located in the termination module was biochemically characterized to be a BVMO tailoring domain that catalyzes the BV oxidation of an acyl carrier protein (ACP)-tethered thioester to an ACP-linked thiocarbonate, which represents the first example of BVMOs operating in cis within the PKS and NRPS biosynthetic paradigm (ACS Catal.2013,3, 444−447). Modular PKSs are well known to use ketosynthase (KS)-driven carbon-carbon bond formation, dehydratase (DH)-mediated dehydration to form double bonds, and product release by thioesterase (TE), all of which are regarded as the “canonical” roles for most polyketide biosynthesis. FR901464 is biosynthesized by a complex acyltransferase (AT)-less PKS system involving a non-terminal TE domain and several mutated KS domains. Now, it was demonstrated that this TE catalyzes the dehydration of the polyketide intermediate to yield a cis-double bond and a mutated KS transfers the nascent polyketide chain with only a cis-double bond to the downstream ACP. This exceptionally rare TE has shown DH function by formation of a cis-double bond as opposed to thioester hydrolysis or macrocyclization (J. Am. Chem. Soc.2014,136, 4488-4491). These findings not only provide new insights of differently enzymatic functions of PKS domains, but also suggest an alternative strategy for cis-double bond formation during the polyketide assembly line. 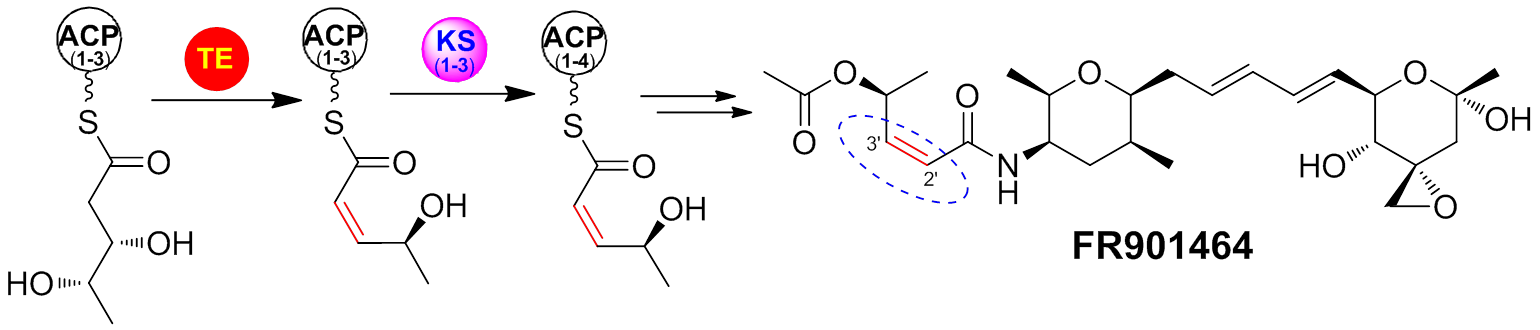
Thioesterase (TE) catalyzes the dehydration of the polyketide intermediate to yield acis-double bond and a mutated ketosynthase (KS) transfers the nascent polyketide chain with only a cis-double bond to the downstream acyl carrier protein. (Imaged by TANG Gong-Li@SIOC) |
|
|


