Palladium-catalyzed oxidative transformations of alkenes are important transformations in organic synthesis. Among them, difunctionalization of alkenes is more attractive due to the high efficiency, and have received much more attention. Recently, a number of palladium-catalyzed difunctionalization of alkenes have been reported by using strong oxidants. One drawback of these reactions is that they usually require strong oxidants, such as PhI(OAc)2, Oxone, NXS, and PhICl2, to generate PdIV complexes, which often produce a large amount of byproducts. Compared to these oxidants, hydrogen peroxide would be preferred because it is inexpensive, environmental benign, and readily available. In the last several years, the research in Guosheng Liu’s group at State Key laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, focused on the difunctionalization of alkenes by using green oxidants. In 2008, Liu and coworkers reported a palladium-catalyzed tandem oxidative cyclization of enynes and chlorination, in which hydrogen peroxide was used as the oxidant, and LiCl as chlorine source. It worth-noted that the reaction appear to proceed via a mechanism involving the oxidation of Csp3–PdII by H2O2, and the formation of C–Cl bonds via a direct reductive elimination pathway that leads to the retention of the stereochemistry of the carbon center (Angew. Chem., Int. Ed. 2008, 47, 5442). Later on, this group found that modified catalytic system could be used to achieve region- and diastereoselectively aminochlorination of alkenes by using palladium catalyst. Again, hydrogen peroxide was used as oxidant and CaCl2 as chlorine source. Preliminary mechanistic study reveals that the reaction involves a reversible aminopalladation step (Chem. Eur. J. 2012, 18, 451). 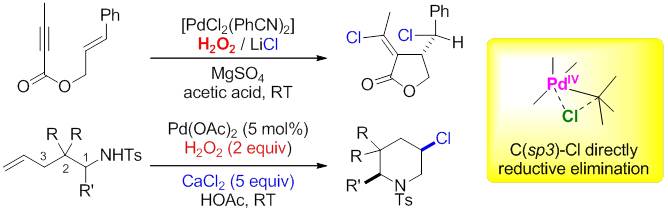
Difunctionalization of alkenes by using green oxidants. (Imaged by LIU guosheng@sioc.) Quite recently, Liu and coworkers developed a palladium-catalyzed intramolecular amino-hydroxylation of alkenes, in which hydrogen peroxide was applied as the sole oxidant (J. Am. Chem. Soc.2014,136, 1766 ). A variety of related alkyl alcohols could be successfully obtained with good yields and excellent diastereoselectivities, which directly derived from oxidation cleavage of alkyl C-Pd bond by H2O2. Facile transformation of these products provided a powerful tool toward the synthesis of 2-amino-1,3-diols and 3-ol amino acids. Preliminary mechanistic studies revealed that major nucleophilic attack of water (SN2 type) at high-valent Pd center contributes to the final C-O(H) bond formation. Overall, above results demonstrated that hydrogen peroxide can be used as green oxidant to survey the possible novel transformation, even in the presence of transition metal catalyst. Further explorations of new reactions are under the progress. 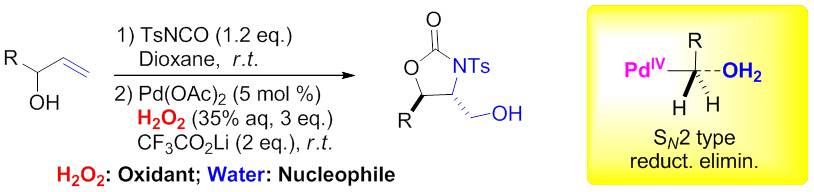
Palladium-catalyzed intramolecular amino-hydroxylation of alkenes by using hydrogen peroxide as the sole oxidant. (Imaged by LIU guosheng@sioc.) All those studies was sponsored by National Science Foundation of China, Ministry of Science and Technology of China, Chinese Academy of Science, and the Science and Technology Commission of the Shanghai Municipality |


