As one of the most lipophilic structural moieties, trifluoromethylthio group (CF3S) has attracted special interests from both academics and pharmaceutical industry. Incorporation of the trifluoromethylthio group into leading drug candidates has become an indispensible, routine strategy for drug discovery, thus resulting in a growing demand that challenges chemists to provide reliable methods for the introduction of this highly valued structural component. In the past several years, Qilong Shen and his coworkers at Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry (SIOC), Chinese Academy of Sciences have been focusing on the development of new reagents and methods for the introduction of the trifluoromethylthio group into small molecules. Previously works from Shen’s lab and Prof. Fengling Qing’s lab on the development of new trifluoromethylation have been highlighted in a cover story in C&E News in 2012. 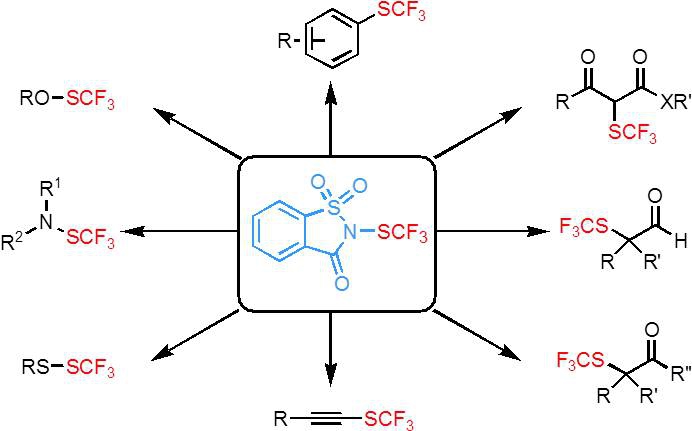
A new electrophilic trifluoromethylthiolating reagent–N-trifluoromethylthiosaccharin. (Imaged by SHEN Qilong@sioc.) The team’s continuing exploration on this topic has led to the invention of a new electrophilic trifluoromethylthiolating reagent–N-trifluoromethylthiosaccharin that makes the introduction of the trifluoromethylthio group much easier (Angew Chem. Int. Ed.2014,53, 9316-9320). N-Trifluoromethylthiosaccharin can be synthesized in two steps from inexpensive artificial sweetener saccharin with AgSCF3 within 30 min. N-trifluoromethylthiosaccharin is a powerful trifluoromethylthiolating reagent that allows the trifluoromethylthiolation of a variety of nucleophiles such as alcohols, amines, thiols, electron-rich arenes, aldehydes, ketones, acyclic b-ketoesters and alkynes under mild conditions. The fast rates, broad scope and tolerance of functionality of these trifluoromethylthiolation reactions made N-trifluoromethylthiosaccharin very attractive as a general reagent that will allow rapid incorporation of the trifluoromethylthio group into small molecules. The work published recently in Angew. Chem. Int. Ed. was highlighted by C&E News titled “Sweet Fluorinating Agent From Saccharin” (C&E News,2014,92(31), 26-27). This work was supported by National Basic Research program of China, National Natural Science Foundation of China and One-Hundred Talent program of SIOC. |


