The [4+2] cycloaddition, in which a 1,3-diene and an alkene react to form two carbon-carbon bonds and construct a cyclohexene, is considered one of the most important transformations in synthetic and natural products chemistry. In nature, however, only a few enzymes are known to possess this activity. These enzymes typically display multiple functions, lack catalytic efficiency, and/or participate in reactions that proceed spontaneously. Access to their biochemical mechanisms is particularly challenging, leaving unsolved for decades the question of whether an enzyme that catalyzes the Diels-Alder reaction, the quintessential type of [4+2] cycloaddition proceeding through a single pericyclic transition state, naturally occurs. On March 2, 2015, the journal Nature Chemical Biology released the finding (Tian, Z., et al.Nat. Chem. Biol.2015, doi: 10.1038/NCHEMBIO.1769) from the Prof. LIU Wen’s group at the Shanghai Institute of Organic Chemistry (SIOC), Chinese Academy of Sciences, which reported that the enzymatic [4+2] cyclization cascade plays an unprecedented central role in the biosynthesis of pyrroindomycins, the pentacyclic spirotetramate natural products that are active against a variety of drug-resistant pathogens. Beginning with a linear intermediate that has two pairs of 1,3-diene and alkene groups, the dedicated cyclases PyrE3 and PyrI4 act in tandem to effectively catalyze the formation of two cyclohexene rings in the dialkyldecalin system and the tetramate spiro-conjugate of the molecules. The two cyclizations, which are individually catalyzed by each stand-alone enzyme, are absolutely enzyme-dependent and proceed in a regio- and stereo-selective manner to complete the enantiomerically pure pentacyclic core. PyrE3 and PyrI4 are thus ideal candidates for a detailed mechanistic examination of a bona fide Diels-Alderase. Their associated cyclization reactions exemplify the cross-bridging paradigm common for a number of structurally relevant natural products, including the cyclohexene-containing spirotetronates that have or lack the dialkyldecalin system. 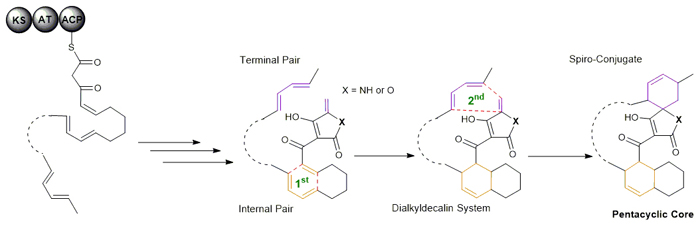
Biosynthetic paradigm to establish two sets of 1,3-diene and alkene groups on a linear unsaturated polyketide intermediate for affording the common pentacyclic scaffold through enzymatic cascade [4+2] cycloaddition reactions. (Imaged by LIU Wen) The generality of coupling or uncoupling these reactions, which explains how nature creates diverse active molecules sharing similar rigid scaffolds, has now been established, indicating the structure-dependent intrinsic nature of [4+2] cyclases in evolution. |


