Chiral cyclic skeletons are ubiquitously found in numerous natural products and drug molecules. Search for efficient and practical asymmetric cyclization reactions has become an important subject in organic chemistry. Professor Wenjun Tang’s research group at State Key Laboratory of Bioorganic & Natural Products Chemistry of Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, have recently made a series of progresses in developing transition-metal catalyzed asymmetric cyclizations that have effectively applied in efficient synthesis of natural products and drug molecules. Dibenzocyclooctadiene lignans are a class of natural products with important biological activities. For example, schizandrin and deoxyschizandrin are two major ingredients of Chinese herbal medicine Wu Wei Zi that provides various medicinal uses such as antitumor agent, liver protective, and tonic. More than 100 dibenzocyclooctadiene lignans have been identified and their syntheses have attracted a great deal of attention. Efficient synthesis of such molecules have been a challenge in organic chemistry due to their unique chiral structures (two contiguous chiral carbon centers and one axial chirality). An efficient strategy would be construction of chiral carbon centers by a catalytic asymmetric cyclization followed by the stereoselective establishment of the axial chirality by an intramolecular coupling. Wenzhen Fu and colleagues have recently developed a highly efficient nickel-catalyzed intramolecular reductive cyclization of alkynones that have led to the formation of a series of tertiary alchols bearing furan/pyran rings in excellent yields and enantioselectivities (Figure 1). The reaction has a broad substrate scope and excellent functional group tolerance. The method has further allowed the efficient synthesis of the lignan dehydroxycubebin as well as the facile construction of chiral dibenzocyclooctadiene skeleton (Angew. Chem. Int. Ed.2015,54, 2520-2524). 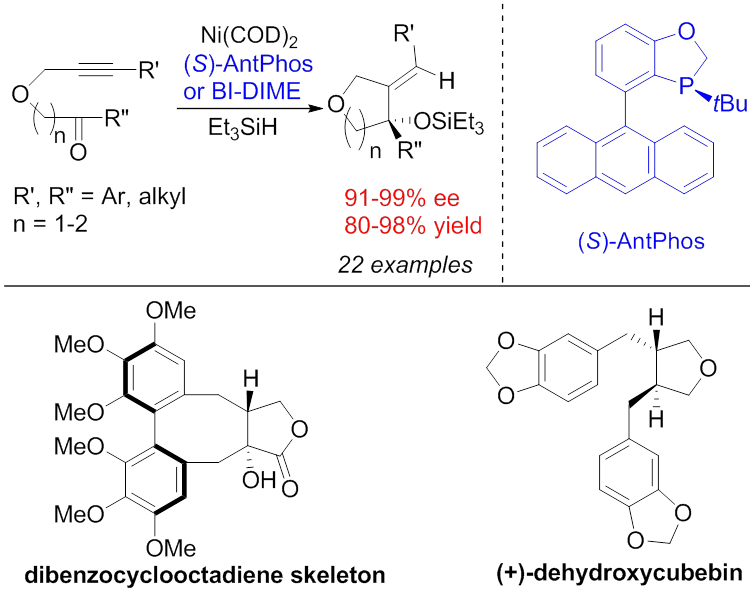
Figure 1. Construction of the chiral dibenzocyclooctadiene skeleton by nickel-catalyzed intramolecular reductive cyclization of alkynones Tricyclic skeletons bearing chiral all-carbon quaternary centers exist in numerous complex terpenes and steroids with interesting biological activities. For example, kaurene is the biosynthetic precursor of plant hormones gibberellins; boldenone is an anabolic steroid; totaradiol is a diterpene natural product that exhibits good antimicrobial properties. The asymmetric intramolecular Heck reaction is a powerful method for constructing chiral polycyclic skeletons bearing all-carbon quaternary centers. However, there are also limitations. Sometimes a number of synthetic steps are required either in constructing the olefin moiety necessary for a heck cyclization or post-modifying the Heck product to the target molecule. Kang Du and colleagues have recently developed a powerful asymmetric palladium-catalyzed dearomative cyclization that has led to a series of chiral phenathrenone derivatives bearing an all-carbon quaternary center in excellent enantioselectivities. The advantages of this cyclization in comparison to the asymmetric Heck reaction include: 1) the substrates are often easy to prepare from simple aromatic compounds; 2) the structure of the cyclization product resembles more to the synthetic target. With this method, the team have successfully accomplished the synthesis of a chiral kaurene intermediate in only 4 steps from simple starting materials, which is much advantageous over a reported 14-step synthesis by asymmetric Heck reaction. The team have also accomplished a facile construction of anabolic steroid boldenone skeleton, and an enantioselective total synthesis of diterpene natural product (-)-totaradiol (Angew. Chem. Int. Ed.2015,54, 3033-3037, hot paper). 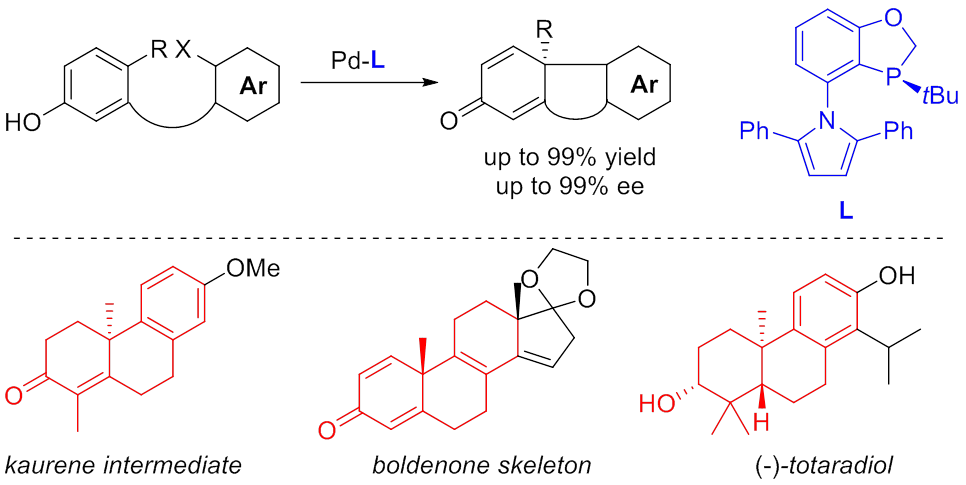
Figure 2. Enantioselective palladium-catalyzed dearomative cyclization for efficient synthesis of terpenes and steroids The projects are financially supported by the “Thousand Plan” Youth program, the National Natural Science Foundations of China, Science and Technology Commission of Shanghai Municipality and Chinese Academy of Sciences. |


