Ferrocenes are of great interest in the fields of material science, organic catalysis, and biomedical research. Particularly, ferrocenes bearing planar chirality have been demonstrated to be highly efficient ligands or catalysts in asymmetric catalysis. Some of them have been widely applied in industrial production. Therefore, the development of efficient methods to introduce planar chirality in the backbone of ferrocene is highly desirable.
The group of Shu-Li You at the State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academic of Sciences, has been involving in the development of asymmetric functionalization of C−H bonds (Asymmetric Functionalization of C–H Bonds,RSC: Cambridge, UK, 2015). In 2013, the identification of palladium acetate and commercially available Boc-l-Val-OH as the catalyst led to the first catalytic asymmetric C−H arylation of dimethylaminomethylferrocenes with aryl boronic acids. Their discovery results in a highly enantioselective synthesis of planar chiral ferrocene compounds (J. Am. Chem. Soc.2013,135, 86). In 2014, they reported an intramolecular C−H arylation of readily available ferrocenes catalyzed by using Pd(OAc)2/(Ra)-BINAP, affording nearly enantiopure planar chiral ferrocenes in excellent yields for a wide range of substrates. The high efficiency of the catalytic system has enabled the reaction at low catalyst loading of 0.5 mol%. Additionally, this methodology could be utilized to synthesize planar chiral P, N-ligand (J. Am. Chem. Soc.2014,136, 4841).
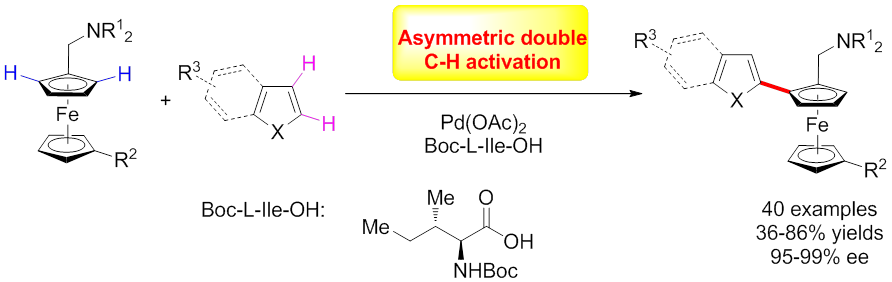
Despite the progresses made in this area, the reported methods require the pre-functionalization of at least one of the coupling partners (either boronic acid or bromide). Recently, a breakthrough has been made in the same laboratory where an asymmetric oxidative cross-coupling reaction was achieved by using Pd(OAc)2 and Boc-l-Ile-OH catalytic system for the first time (J. Am. Chem. Soc.2016,138, 2544). Planar chiral ferrocenes were synthesized with high yields and enantioselectivity directly from dimethylaminomethylferrocenes and electron-rich heteroarenes via a twofold C−H bond activation pathway that takes place without having to prefunctionalize either coupling partner. The atom-economical reaction proceeds using oxygen in the air as a green oxidant instead of a metal oxidant, which is in accordance with the principle of green chemistry. Notably, nearly enantiopure planar chiral ferrocene derivatives were synthesized with exclusive regioselectivity. This methodology provides an efficient approach to the synthesis of planar chiral ferrocene derivatives, which is of great significance to design and synthesize planar chiral ligands or catalysts. This work was recently highlighted by Chemical & Engineering News with a title of “Dual C–H/C–H asymmetric cross-coupling unveiled” on March 14, 2016.
These projects were sponsored by the National Natural Science Foundation of China, Ministry of Science and Technology of PRC, Chinese Academy of Sciences, and Science and Technology Commission of Shanghai Municipality.


