Carbon-heteroatom bond formation is one of the most important transformations in organic synthesis. Based on an analysis of chemical reactions currently used in medicinal chemistry, it was found that alkylation and arylation of heteroatoms is the most popular transformation and only two reactions that were developed in recent 30 years are frequently used in medicinal chemistry. They are Suzuki-Miyaura reaction (for C-C bond formation), and Buchwald-Hartwig reaction (for arylation of heteroatom-containing nucleophiles). Although Buchwald-Hartwig reaction has been found a great number of synthetic applications, it still has drawbacks such as using very expensive metal complexes and ligands and toxicity issue of palladium.
Recently, Dawei Ma’s group at SIOC reported that some very cheap and conveniently available N-aryl-N’-alkyl substituted oxalamides are powerful ligands to promote Cu-catalyzed coupling between (hetero)aryl chlorides and phenols, leading to the formation of a great variety of diaryl ethers in good to excellent yields at 120 oC (Angew. Chem. Int. Ed.2016,55, 6211). A board range of functional groups are tolerated, which include amines, alcohols, amides, halides, esters, ketones, nitriles, as well as a number of heterocycles. This new catalytic system could be used for preparing insecticides diafenthiuton, permethrin, cypermethrin and deltamethrin from suitable aryl chlorides, and thereby providing a more economic approach for manufacturing these chemicals.
This work is an extension of the studies on copper-catalyzed coupling reactions from the same group. Last year, Ma and coworkers revealed that some N,N’-diaryl substituted oxalamides were effective ligands for copper-catalyzed aryl amination with (hetero)aryl chlorides (J. Am. Chem. Soc. 2015, 137, 11942; Org. Lett. 2015, 17, 5934). Since substituted aniline and diaryl ether are common moieties for a large number of pharmaceutical products, agrochemicals and materials, these newly developed reactions will definitely find applications in producing the functional molecules, which represents a big breakthrough in the area of metal-catalyzed coupling reactions.
These projects were sponsored by the National Natural Science Foundation of China and Chinese Academy of Sciences.
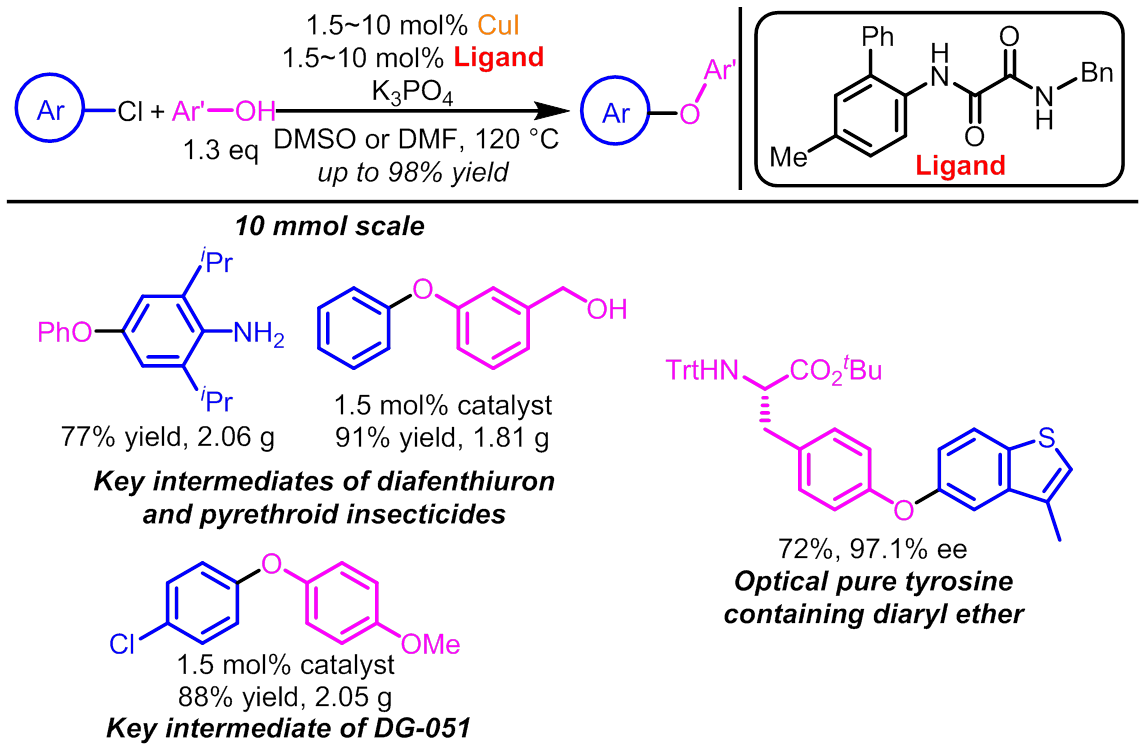
Powerful CuI/Oxalamide Catalytic System for Efficient and Scalable Diaryl Ether Formation from (Hetero)aryl Chlorides and Phenols.(image by MA Dawei)


