Diels-Alder [4+2] cycloaddition, one of the most famous organic name reactions, is widely used in synthetic chemistry, and can serve as a kind of click chemistry applied to material and biological science. Up to now, a variety of organic small molecule-based catalysts have been developed for accelerating D-A reactions and controlling the stereoselectivity. According to Pauling’s theory, that is to say enzymes can stabilize the transition states of the reactions, biomacromolecule-based catalysts for D-A reactions (e.g., abzymes and ribozymes) were also prepared via directed evolution. However, whether and how naturally occurring enzymes can catalyze this type of reaction is still not completely clear.
Recently, Dr. Wen Liu’ group at SIOC together with Dr. Lifeng Pan’s group reported the structure and detailed catalytic mechanism of PyrI4, a monofunctional Diels-Alderases (DAases) involved in the biosynthetic pathway of pyrroindomycins (PYRs). This work was published on Cell Chemical Biology (Cell Chem. Biol.,2016,23, 352-360.) and highlighted by Prof. Hideaki Oikawa, who is one of the world pioneers studying DAases, on the same journal (Cell Chem. Biol.,2016,23, 429-430.).
In this paper, Liu group and Pan group report the crystal structures of PyrI4 alone and in complex with its product. Comparative analysis of the structures, combined with biochemical analyses, lead them to propose a unique trapping mechanism whereby the lid-like action of the N-terminal tail imposes conformational constraints on the b barrel catalytic core, which enhances the proximity and polarization effects of reactive groups (1,3-diene and alkene) to drive cyclization in a regio- and stereospecific manner. They found that PyrI4 functions as an exo-selective DAase by trapping the flexible substrate with the dynamic N-terminally extended sequence, synergistically constraining its conformation to an exo transition state structure in the rigid β-barrel core, accelerating the reaction through an electron-withdrawing effect on dienophile, and finally, releasing the rigidified product and avoiding its inhibition effect for cycling on the change in chemical conformation. The elucidation of this unique enzymatic process for the first time reveals the nature’s strategy for catalyzing a Diels-Alder reaction.
The experimental section of this work is mainly carried out by Qingfei Zheng and Jujiao Guo, and Dr. Wen Liu together with Dr. Lifeng Fan direct the research. This work was supported in part by grants from MST, Thousand Talents Program, STCSM, SRSSA, NSFC of China.
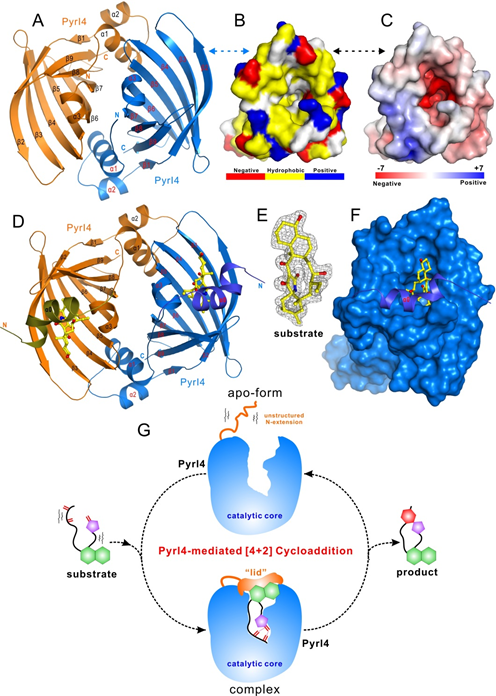
The structures of PyrI4 (A, B and C) and that in complex with the substrate (D, E and F); the cartoon of the catalytic cycle and mechanism of PyrI4 (G).(image by LIU Wen)


