Autophagy is a highly regulated and lysosome-dependent cellular metabolic process involving in the degradation of mis-folded bulk protein aggregates, damaged organelles and invading pathogens in eukaryotic cells. The dysfunction of autophagy is associated with many neurodegenerative diseases. Optineurin(OPTN) is a multifunctional autophagy receptor, which plays important roles in the cargo-recognition steps during several selective autophagy processes. The function of OPTN in selective autophagy is regulated by the TBK1 kinase, and mutations of OPTN and TBK1 are associated with primary open-angle glaucoma (POAG) and amyotrophic lateral sclerosis (ALS), two progressive neurological disorders. However, until now, the structural basis of the optineurin and TBK1 interaction and the related gene mutation-caused disease mechanism of optineurin and TBK1 are still unclear. Recently, Dr. Lifeng Pan’ group at SIOC reported the crystal structures of optineurin/TBK1 complex and the related NAP1/TBK1 complex. This work was published on the Nature Communications journal (Nature Communications, 2016, DOI: http://www.nature.com/articles/ncomms12708). In this study, they firstly minimized the OPTN and TBK1 fragments which are essential for the interaction using biochemical approaches. And then, they solved the crystal structures of the OPTN/TBK1 complex and the related TBK1/NAP1 complex through the X-ray crystallography. The determined complex structures not only uncover the detail molecular mechanism of the interactions of TBK1 with OPTN and NAP1, revealing a general binding mode between TBK1 and its associated adaptor proteins, but also provide the structural explanations for the molecular mechanism of POAG- associated OPTN E50K missense mutation and ALS-associated TBK1 E696K mutation. In addition, a series of biochemical and cellular experiments demonstrated that the glaucoma-associated optineurin E50K mutation not only enhances the interaction between optineurin and TBK1 but also alters the oligomeric state of optineurin, and the ALS-related TBK1 E696K mutation specifically disrupts the optineurin/TBK1 complex formation but has little effects on the NAP1/TBK1 complex, in cells. Thus, their study, firstly, elucidated the structural basis for the regulation mechanism of optineurin-involved selective autophagy processes by TBK1, and provided mechanistic insights into those currently known disease-causing optineurin and TBK1 mutations found in human patients. 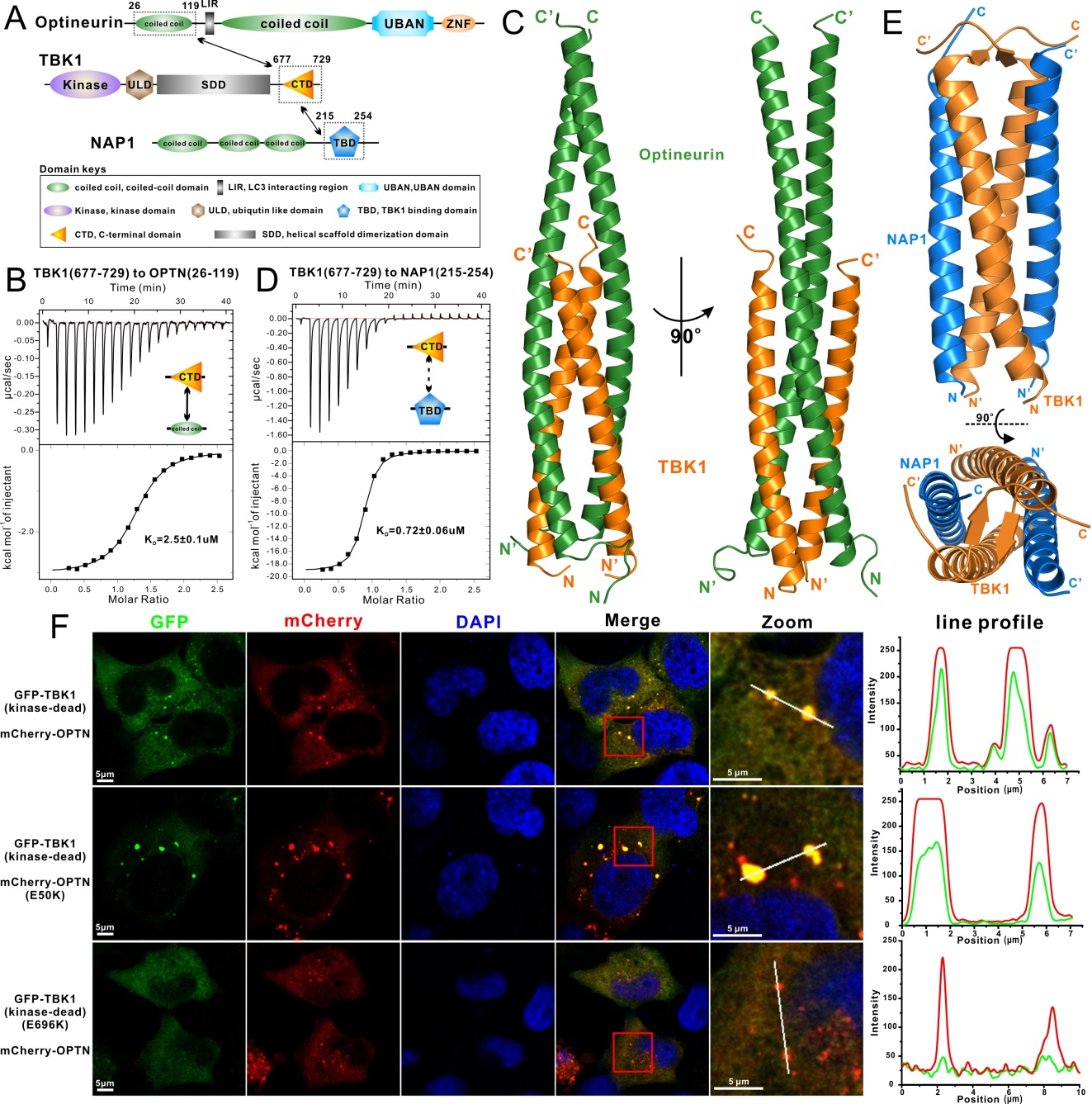
Figure (A) A schematic diagram showing the domain organizations of OPTN, TBK1 and NAP1 proteins. (B) ITC-based measurement of the binding affinity of TBK1 CTD with the OPTN(26-119) fragment. (C) The overall structure of OPTN/TBK1 complex. (D) ITC-based measurement of the binding affinity of TBK1 CTD with the NAP1(215-254) fragment. (E) The overall structure of NAP1/TBK1 complex. (F) The cellular co-localization analyses of OPTN or OPTN E50K mutant with TBK1, as well as OPTN with TBK1 E696K mutant in HeLa cells.(Imaged by PAN Lifeng) This work was supported by grants from the National Natural Science Foundation of China (31470749), National Basic Research Program of China (2013CB836900 and 2016YFA0501900), a “Thousand Talents Program” young investigator award, the start-up fund from State Key Laboratory of Bioorganic and Natural Products Chemistry and Chinese Academy of Sciences. Contact: Prof. PAN Lifeng Shanghai Institute of Organic Chemistry (SIOC), Chinese Academy of Science E-mail: panlf@sioc.ac.cn |


