As a continuous effort to develop mild conditions for copper-catalyzed arylation with nucleophiles, Dawei Ma’s group at SIOC recently discovered that combination of Cu(acac)2 and BHMPO (N,N’-bis(4-hydroxy-2,6-dimethylphenyl)-oxalamide) is a powerful catalytic system for hydroxylation of (hetero)aryl halides (J. Am. Chem. Soc., 2016, http://dx.doi.org/10.1021/jacs.6b08114). Carbon-heteroatom bond formation is one of the most important transformations in organic synthesis. Based on an analysis of chemical reactions currently used in medicinal chemistry, it was found that alkylation and arylation of heteroatoms is the most popular transformation. Palladium and copper catalyzed coupling reactions are major tools for creating such carbon-heteroatom bonds. Although Cu-based catalysts are rather cheaper than Pd-based catalysts, they suffer from poor reaction scope and using high catalytic loadings. In their previous studies (Figure 1), Ma and coworkers discovered that a series of N,N’-disubstituted oxalamides are extremely effective ligands for promoting Cu-catalyzed coupling reactions of aryl chlorides and nucleophiles, leading to the formation of aryl amines and diaryl ethers under relatively mild conditions. (J. Am. Chem. Soc. 2015, 137, 11942; Org. Lett. 2015, 17, 5934; Angew. Chem. Int. Ed. 2016, 55, 6211). 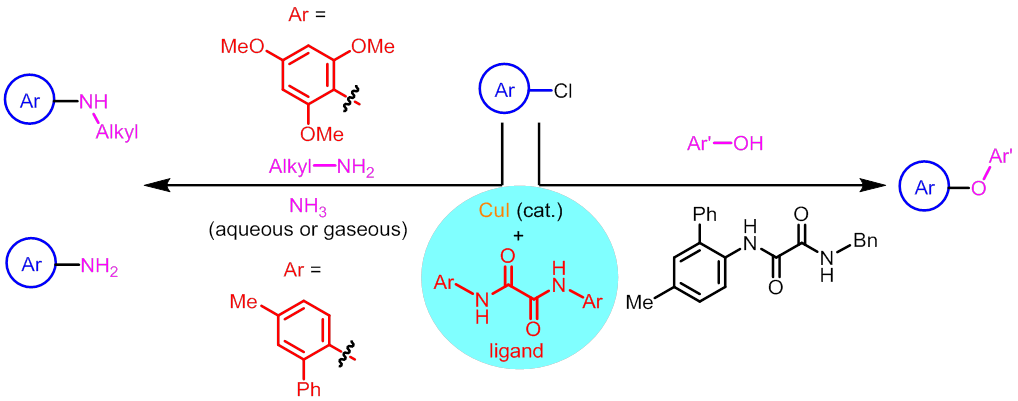
Figure 1. Copper/oxalamides-promoted C-N and C-O coupling reactions with aryl chlorides In their recent report, Ma and coworkers described that BHMPO, is a very efficient oxalamide ligand for copper-catalyzed hydroxylation of (hetero)aryl halides (Figure 2). For less reactive aryl chlorides, the coupling reaction occurred at 130 oC under the catalysis of Cu(acac)2/BHMPO to provide phenols and hydroxylated heteroarenes. A broad range of electron-rich and electron-poor aryl chlorides, and heteroaryl chlorides are compatible with these conditions, delivering the corresponding products in good to excellent yields. When more reactive aryl bromides and iodides were used, catalytic loadings for both copper salt and ligand could be reduced to 0.5 mol% and the reaction proceeded smoothly at 40-80 oC. It is notable that the catalytic loadings could be further reduced and the reaction time could be even shorter in a scaled-up experiment. For example, the hydroxylation of 1-(3-chlorophenyl)-ethanone on a 20 mmol scale completed in 24 h by using only 2 mol % catalyst and ligand, while a complete conversion was observed in 12 h when the hydroxylation of 3-bromo-1-iodobenzene was run on a 20 mmol scale. Phenols, hydroxylated heteroarenes, and their derivatives are important structural constituents of pharmaceuticals, agrochemicals, polymers, and natural products. Their new catalytic system can be used for hydroxylating relatively inexpensive (hetero)aryl chlorides, and make hydroxylation of aryl bromides and iodides much more convenient. This study provides a valuable approach for preparing phenols and hydroxylated heteroarenes, particularly for large-volume production in the future. 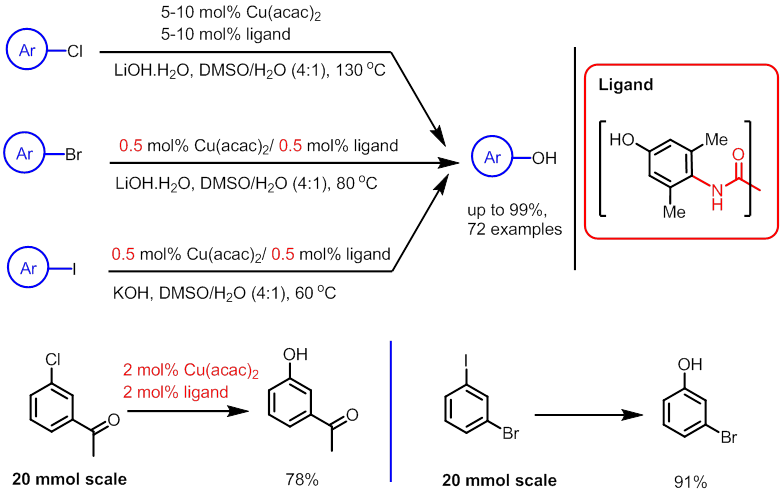
Figure 2 Cu(acac)2/BHMPO-catalyzed hydroxylation of (hetero)aryl halides These projects were sponsored by Chinese Academy of Sciences and National Natural Science Foundation of China. |


