Thiopeptide is a kind of typical ribosomally synthesized and post-translationally modified peptides (RiPPs), which has more than 100 members. Biosynthesis studies of thiopeptides not only contribute to the development of new drug seeds, but also facilitate the understandings in complex post-translational modifications (PTMs) of proteins and/or peptides. Among all the thiopeptide members, thiostrepton (TSR) possesses the most complex structure and diverse biological activities, which draws researchers’ great intersects. Recently Dr. Wen Liu’s group at SIOC elucidated a key reaction involved in the construction of TSR side-ring system, which is mediated by a unique dual functional α/β-hydrolase fold enzyme. This work has been published on the journal of Proceedings of the National Academy of Sciences of the United States of America (Proc. Natl. Acad. Sci. USA.,2016, DOI: 10.1073/pnas.1612607113.)
In the previous work, Liu’s group developed a strategy named precursor-directed mutational biosynthesis to generate derivatives of thiostrepton. Using this strategy, they successfully obtained several biological activity-improved TSR derivatives (Org. Chem. Front., 2015, 2, 106-109; Org. Chem. Front., 2016, 3, 496-500.), and discovered a novel dual mode of action of thiostrepton against intracellular pathogens by utilizing some of these derivatives as chemical probes (Chem. Biol., 2015, 22, 1002-1007.). Based on these results, they proposed that the quinaldic acid (QA) moiety-containing side-ring system of TSR is biologically relevant but tunable.
In recent studies, they intended to produce more thiostrepton derivatives through the same method, but unexpectedly a new shunt product without outstanding biological activities was obtained. This unusual result promoted them to pay more attention to the genes probably responsible for the oxidative modifications on the thiostrepton skeleton. Combining gene inactivation, exogenous chemical feeding, and in vitro assays, they assigned the functions of two cytochromes P450 in thiostrepton biosynthesis (ACS Chem. Biol., 2016, 11, 2673-2678.). This shows that precursor-directed mutational biosynthesis can not only be used to expend molecular diversities but also be used to probe complex biosynthetic logics.
In this work, Liu’s group isolated and identified a key intermediate using the same strategy. This side-ring open and epoxy QA moiety-containing compound (6′-fluoro-7′, 8′-epoxy-TSR) was accumulated when they fed 6-F-QA as a precursor into the mutant strain △tsrT for generating the target product 6’-fluoro-TSR. To find the enzyme responsible for this intriguing ring-closure reaction, they reanalyzed the biosynthetic gene cluster of TSR and found a functionally unassigned gene tsrI coding for an α/β-hydrolase fold protein TsrI. Combining in vivo and in vitro assays, they proved that TsrI exhibits a dual activity for endopeptidyl hydrolysis of the leader peptide and epoxide ring opening/macrocyclization of TSR side ring. These two functions of TsrI rely on the same catalytic triad (Ser-His-Asp) that is highly conserved in the superfamily of α/β-hydrolase fold enzymes. These findings not only answer the question how the side ring of TSR is constructed, but also facilitate the understandings of the biochemistry of α/β-hydrolase fold protein superfamily, thereby shedding light on the future studies of synthetic biology-based molecular engineering of bimacrocyclic thiopeptide antibiotics. 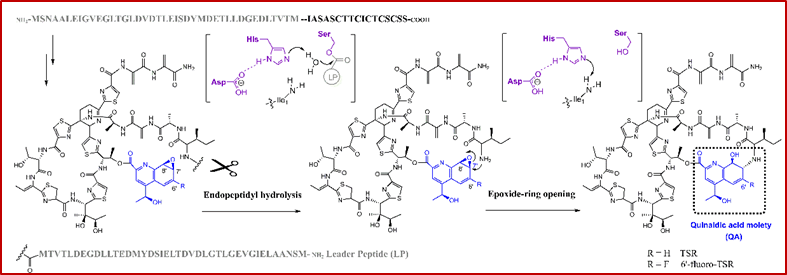 Dual activity of an α/β–hydrolase fold protein for cascade C-N bond cleavage and formation in thiostrepton biosynthesis. (Imaged by LIU Wen)
The experimental sections of aforementioned work are all mainly carried out by the PhD. candidate Qingfei Zheng and the associate professor Dr. Shoufeng Wang, and Prof. Wen Liu directs the research. This work was supported in part by grants from NSFC, STCSM, CAS and MST of China.
Contact: Prof. LIU Wen Shanghai Institute of Organic Chemistry (SIOC), Chinese Academy of Science E-mail: wliu@mail.sioc.ac.cn |


