Palladium-catalyzed C–H funtionalization reaction is a powerful chemical transformation. And reductive elimination from metal centers is a key step in a myriad of carbon–carbon and carbon–heteroatom bond–forming reactions. However, reductive elimination reactions from Pd(II) center to form C–O, C–N, or C–F bonds are typically sluggish. One strategy for promoting otherwise challenging reductive elimination reactions is to oxidize the metal center using an external chemical oxidant. Unfortunately, these oxidants have drawbacks in that they produce undesired byproducts, have poor atom economy, or are expensive. Thus, the development of novel oxidation systems represents a central challenge in the field of oxidative Pd(II)-catalyzed C–H functionalization. Mei group from Shanghai Institute of Organic Chemistry has successfully developed Pd(II)-catalyzed C(sp3)–H functionalization reactions via electrochemical oxidation (J. Am. Chem. Soc.,2017,139, 3293–3298). This catalytic system utilizes electric current to oxidize Pd(II) species to yield high-valent PdIII or PdIV species in the presence of the spectator counteranion, which is then incorporated into the product through reductive elimination. This catalytic system offers several advantages over traditional methods: 1) it obviates the use of dangerous and toxic reagents; 2) it results in better chemo-selectivity owing to controllable electrolysis; 3) it provides unique platform for mechanistic study. This work was financially supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant XDB20000000), “1000-Youth Talents Plan”, National Science Foundation (NSF) of China (Grant 21421091, 21572245), and Shanghai Science and Technology Committee (S&TCSM) (Grant 15PJ1410200). 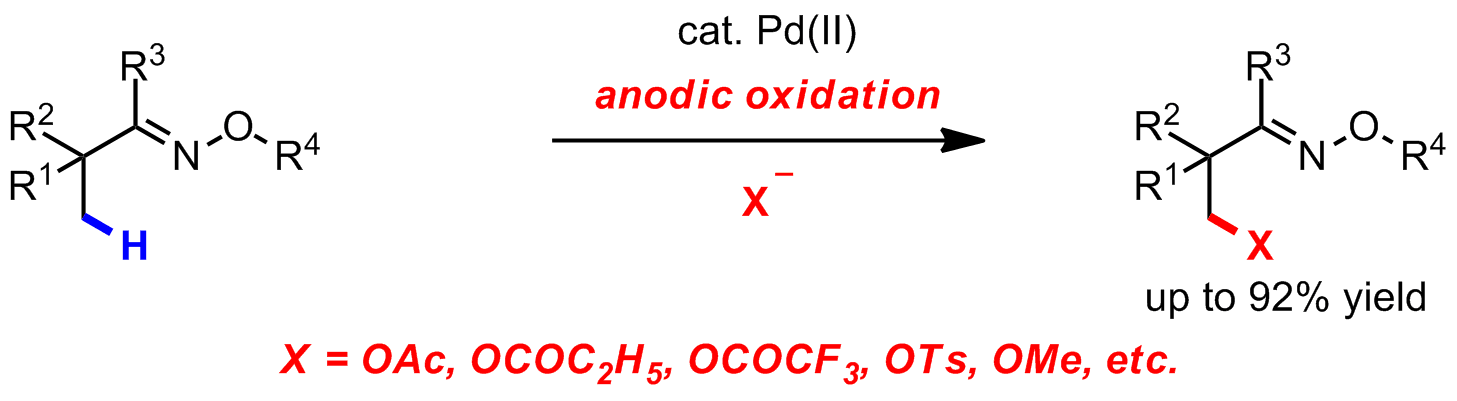
Palladium-catalyzed C(sp3)-H oxygenation via electrochemical oxidation ( imaged by MEI Tian-Sheng@SIOC) Contact: Prof. MEI Tian-Sheng Shanghai Institute of Organic Chemistry (SIOC), Chinese Academy of Science E-mail: mei7900@sioc.ac.cn |


