Initially isolated by Renxiang Tan and co-workers from mantis-associated fungus Daldinia eschscholzii, Dalesconol A and B are two unusual polyketides that exhibit strong immunosuppressive activities comparable to that of clinically used cyclosporine A, but with superior selective index values for their noncytotoxic nature. Interestingly, both natural dalesconol A and B are scalemic mixtures with an excess of their (-)-enantiomers (ratios of (-)/(+) ≈ 2/1). Intriguingly, both scalemic mixtures of natural dalesconol A and B provided more potent immunosuppressive activities than their enantiomers. Structurally, dalesconol A and B possess an architecturally unique and highly dense carbon skeleton containing seven fused rings of various sizes and two stereogenic centers including one sterically congested all-carbon quaternary center. All these features make dalesconol A and B attractive synthetic targets. The only total syntheses of racemic dalesconol A and B were accomplished by Snyder and co-workers through a sequence of 15 linear steps and 25 overall steps. The Wenjun Tang research group at Shanghai Institute of Organic Chemistry are interested in developing efficient catalytic coupling reactions for the concise syntheses of biological interesting natural products and drugs. Two years ago, they developed a practical and enantioselective palladium-catalyzed dearomative cyclization for the efficient construction of a series of chiral phenanthrenone derivatives bearing an all-carbon quaternary center. The strategy was applied successfully for the efficient synthesis of terpenes and steroids. Recently, Guoqing Zhao, Guangqing Xu and Chao Qian accomplished the first enantioselective syntheses of (+)-dalesconol A and B within 9 steps and 11% overall yield from commercially available starting material (J. Am. Chem. Soc.2017,139, 3360-3363), which features an unprecedented enantioselective palladium-catalyzed dearomative cyclization?kinetic resolution cascade to install the sterically congested chiral all-carbon quaternary center, an effective sterically hindered Stille coupling, a powerful DDQ oxidation to furnish all requisite unsaturation, and a tandem hydrolysis?Michael addition ring closure sequence. The research work will facilitate the discovery and the development of new immunosuppressants. 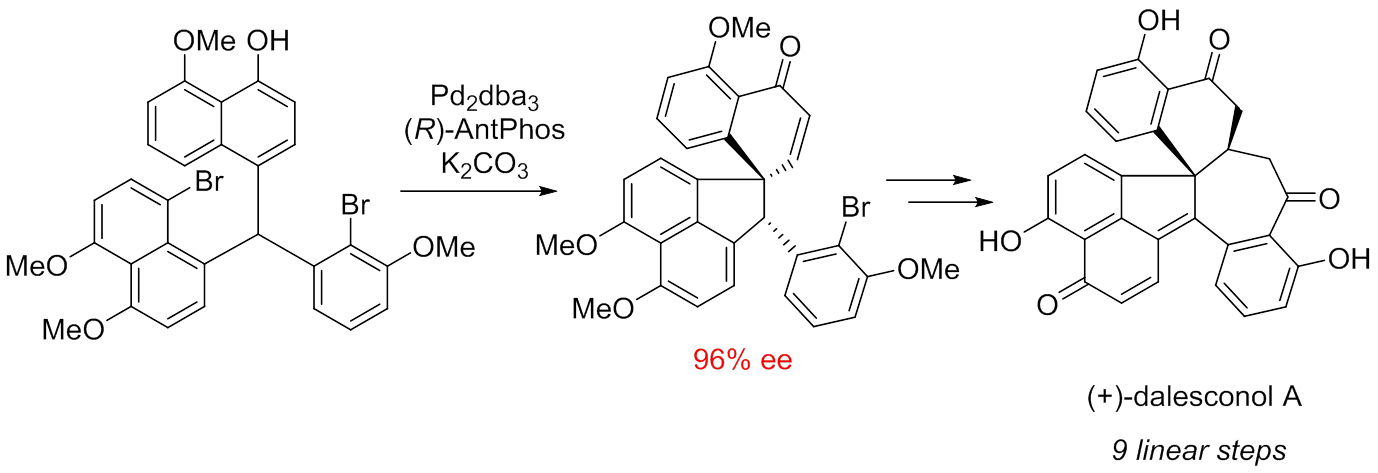
Concise synthesis of (+)-dalesconol A (Image by Wenjun Tang) The work is supported by the National Natural Science Foundation of China, Strategic Priority Research Program of the Chinese Academy of Sciences, and State Key Laboratory of Bio-Organic and Natural Products Chemistry. Contact Wenjun Tang Shanghai Institute of Organic Chemistry, CAS E-mail: tangwenjun@sioc.ac.cn |


