Due to the unique properties of fluorine atom(s), fluorinated compounds play important roles in agrochemicals, pharmaceuticals, and materials science. Over the past decade, impressive achievements have been made in efficiently introduction of fluorinated group into organic molecules. However, most of these approaches used well-known and expensive fluorinating reagents, the use of inexpensive, abundant and widely available industrial raw material fluoroalkanes has received less attention because of their relatively inert reactivities. Most recently, Xingang Zhang group from Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, reported the first example of direct formation of difluoromethylated arenes from inexpensive and widely available industrial raw material ClCF2H (Nature Chemistry,2017, DOI: 10.1038/NCHEM.2746) (Figure 1 b-c). Dr. Zhang Feng, Qiao-Qiao Min and Xia-Ping Fu are the coauthors for this paper. CF2H is not only considered as a bioisostere of a hydroxy and thiol group, but also functions as a lipophilic hydrogen bond donor. The selective introduction of CF2H onto aromatic rings can significantly improve their bioactivities compared with their non-fluorinated counterparts. Thus, difluoromethylation is a useful strategy for the modification of biologically active compounds. However, the difluoromethylating reagents used in previous work are expensive and require multistep synthesis. ClCF2H (R22) is the most inexpensive and abundant industrial raw material used for the production of various fluorinated polymers (i.e. polytetrafluoroethylene, PTFE) (Figure 1a). From the point view of cost-efficiency and step-economy, ClCF2H would be an ideal and straightforward difluoromethylating reagent, however, the use of ClCF2H for difluoromethylation of aromatics remains a challenge and has not been reported. 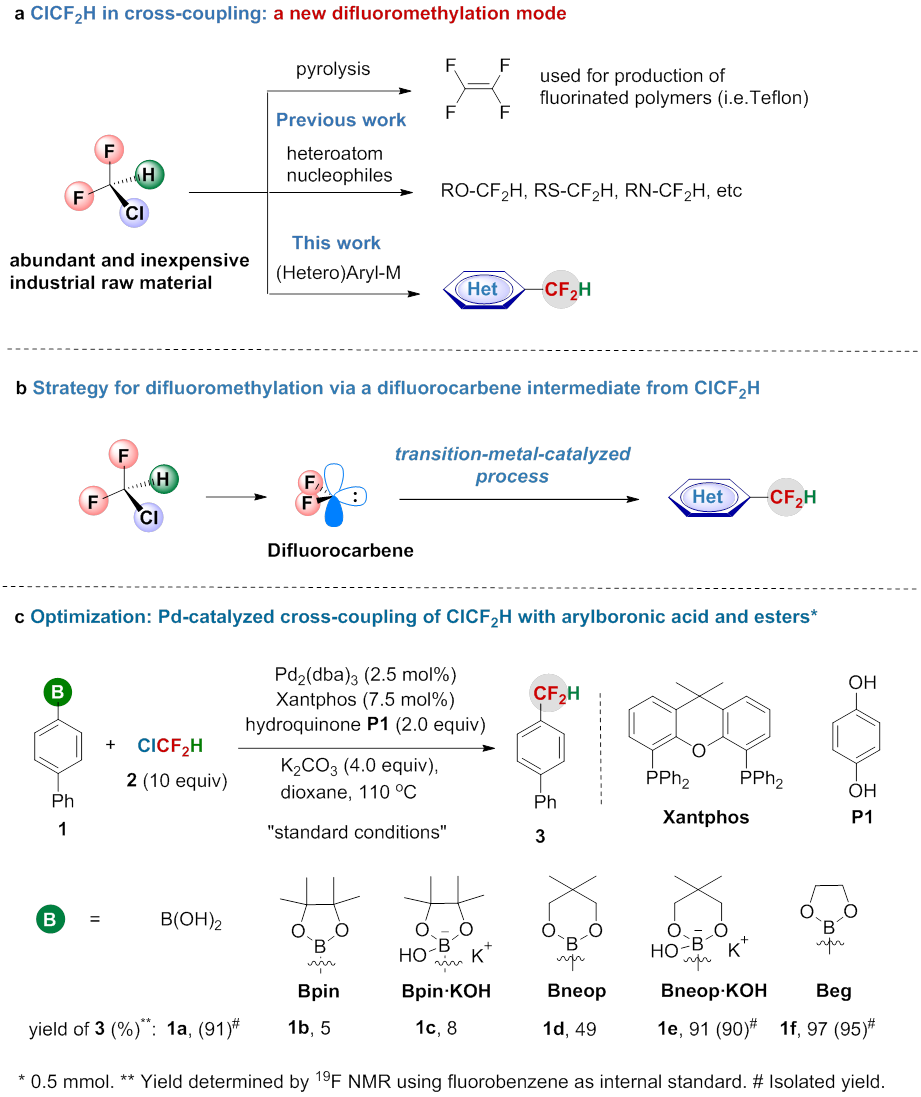
Figure 1. Activation of ClCF2H in organic synthesis. (imaged by ZHANG Xingang) Zhang’s group discovered the first example of Pd-catalyzed difluoromethylation of arylboronic acids with bromodifluoroacetate via a difluorocarbene intermediate (Org. Lett. 2016, 18, 44) on the basis of their previous work (J. Am. Chem. Soc. 2010, 132, 4506; Angew. Chem. Int. Ed. 2014, 53, 1669). Although several difluorocarbene metal complexes have been isolated, the use of metal difluorocarbene to catalyze the reaction remains a great challenge. Inspired by their previous work, they have developed the first example of palladium-catalyzed difluoromethylation of (hetero)arylborons with ClCF2H through a transition-metal difluorocarbene pathway, which provides a new mode for activation of ClCF2H (Figure 1b-c). The reaction features several advantages: 1) high efficiency with broad substrate scope; 2) low-cost difluoromethylating reagent; 3) excellent functional group tolerance, even towards heteroaromatics and biologically active molecular complexes. Thus, this convenient approach provides a useful tool for the modulation of drugs and biologically active molecules (Figure 2). Current efforts are devoted to elucidating the detailed mechanism and to lower the loading amount of catalyst and ClCF2H. 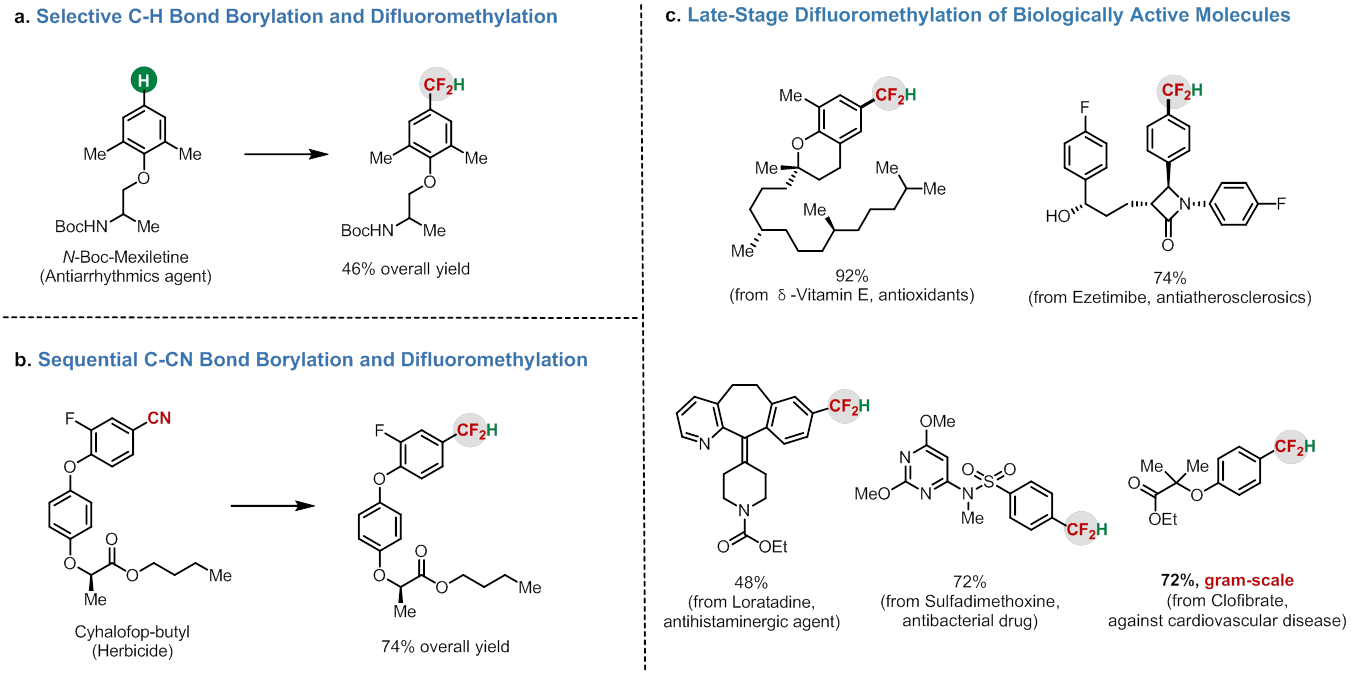 Figure 2. Late-stage difluoromethylation of biologically active molecules. (imaged by ZHANG Xingang) Financial support for this work was provided by the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, Shanghai Institute of Organic Chemistry and Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, CAS.
Contact:
ZHANG Xingang Shanghai Institute of Organic Chemistry , CAS E-mail: xgzhang@sioc.ac.cn |


