The carbonyl group is an important functional group in organic molecules. Comparing to many synthetic efforts to construct carbonyl-C(sp3) (OC-alkyl) bonds, it is challenging to cleave the OC-alkyl bond selectively. The alkoxyl radical is a versatile reactive intermediate for carbon-carbon bond cleavage reactions, however the applications on the OC-alkyl bond cleavage is unknown. Yiyun CHEN's group from Shanghai Institute of Organic Chemistry at the Chinese Academy of Sciences recently discovered that cyclic iodine reagents activated cyclic and liner alcohols effectively to generate alkoxyl radicals. Moreover, the different derivatives of cyclic iodine reagents bear different alcohol activation reactivity, which enabled b-amide, b-ester, and b-ketone alcohols for the synthesis of ynamides, ynoates, and ynones (Angew. Chem., Int. Ed.2017, 56, 2478–2481). This work was financially supported by the National Basic Research Program of China, Strategic Priority Research Program of the Chinese Academy of Sciences, and National Science Foundation of China. 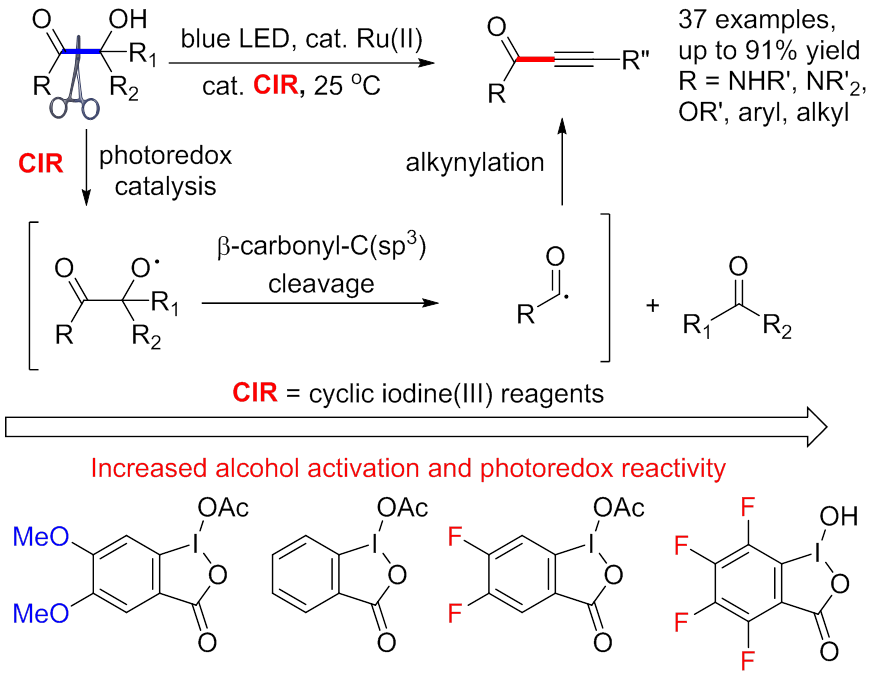
Hypervalent iodine reagents enable selective carbonyl-C(sp3) bond cleavage via photoredox catalysis (Image by CHEN) Contact Author:Yiyun Chen Shanghai Institute of Organic Chemistry , CAS E-mail: yiyunchen@sioc.ac.cn |


