Multi aminoacyl-tRNA synthetase complex (MSC) is the second largest machinery for protein synthesis in human cells and also regulates multiple nontranslational functions such as cancer metastasis, inflammation, allergic response, etc. New research from Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, published in theJournal of Biological Chemistry, shows that the retractility of the complex may be critical for its physiological functions.
Pengfei Fang, who oversaw the work, is a professor of the State Key Laboratory of Bioorganic and Natural Products Chemistry. He studies the molecular mechanism of the assembly of the mammalian MSC over the past years. The mammalian MSC contains nine different cytoplasmic aminoacyl-tRNA synthetases, and accounts for the translation of nearly half of the genetic codes through catalyzing the aminoacylation of tRNA moleculs in cells. Within MSC, the lysyl-tRNA synthetase (LysRS) associated with the scaffold protein AIMP2, is not only essential for protein translation but also plays critical roles in allergic response, tumor migration, HIV infection, and other aspects.
It is to be expected that when LysRS performs translational and nontranslational functions, it might need different structural states in MSC for fine regulation. With a crystal structure at 1.88? resolution, the new paper shows that LysRS can make a tight X-form assembly with the scaffold AIMP2 protein. The novel X-form assembly co-exists with the previously discovered loose V-form assembly in human cells (Figure 1). The two forms of the complex structure may reflect different stages of LysRS function.
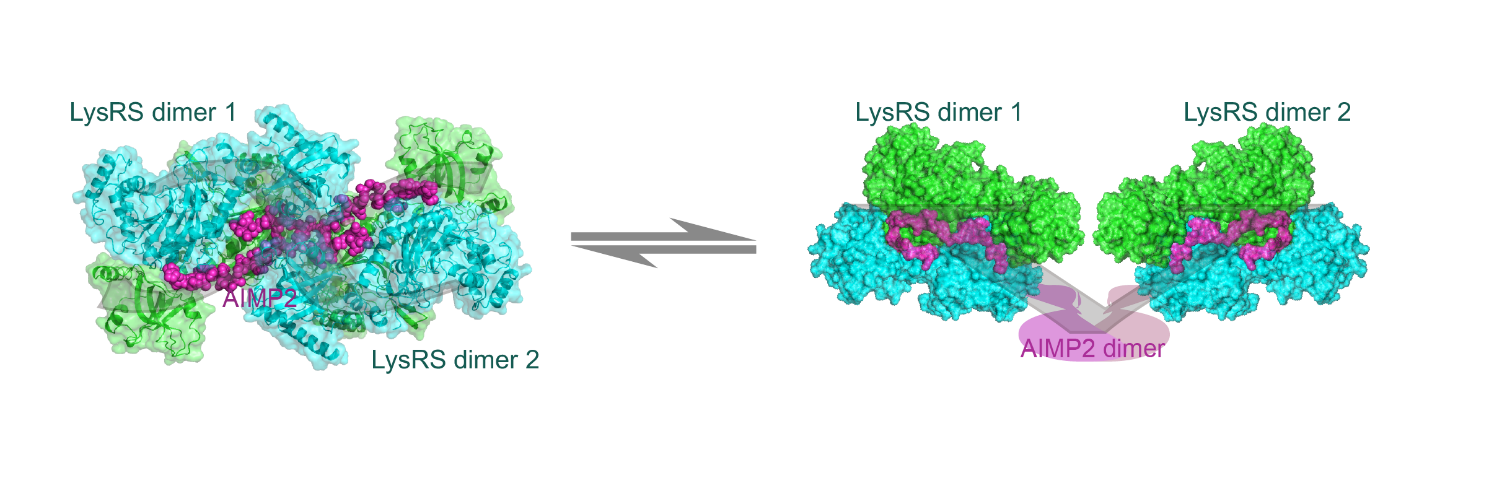
Figure 1. The two forms of LysRS-AIMP2 sub-complexes: X-form (left) and V-form (right) (Imaged by FANG Pengfei@SIOC)
The AIMP2 molecule contains two LysRS-binding motifs at its N terminus. The two tandem motifs are crucial in concatenating two LysRS dimers into the compact X-form assembly. In the X-form, four tRNA molecules can be docked simultaneously on the structure without any clashes, suggesting a functional state for efficient protein translation. A few mutations of LysRS have been discovered in human patients in recent years. The complex model provides a new direction for understanding the underlying pathogenesis of these diseases.
The study was funded by the Chinese Academy of Sciences, National Natural Science Foundation of China, Shanghai Institute of Organic Chemistry, and the State Key Laboratory of Bioorganic and Natural Products Chemistry.
Contact
Author:FANG Pengfei
Shanghai Institute of Organic Chemistry, CAS
E-mail: FangPengfei@sioc.ac.cn


