In a latest study published on March 29th in J. Biol. Chem., a research group led by Professor Cong Liu of the Institute of Organic Chemistry, Chinese Academy of Sciences, successfully identified and characterized a novel chaperone activity of Hsp104 which plays an essential role in preventing pathological protein aggregation in different neurodegenerative diseases.
A variety of devastating neurodegenerative diseases including Alzheimer’s (AD) and Parkinson’s (PD) are closely associated with protein amyloid aggregations which are also served as the pathological hallmark of the related diseases. Hsp104, a conserved hexameric AAA+ protein, can disaggregate, in an ATP-dependent manner, the toxic aggregates formed by diverse amyloid proteins. Hsp104 is protective in various cellular and animal models associated with amyloid-related diseases, and provides a potential therapeutic candidate for amyloid diseases. Therefore, it is important to understand the structural basis underlying the interplay between Hsp104 and pathogenic amyloid. 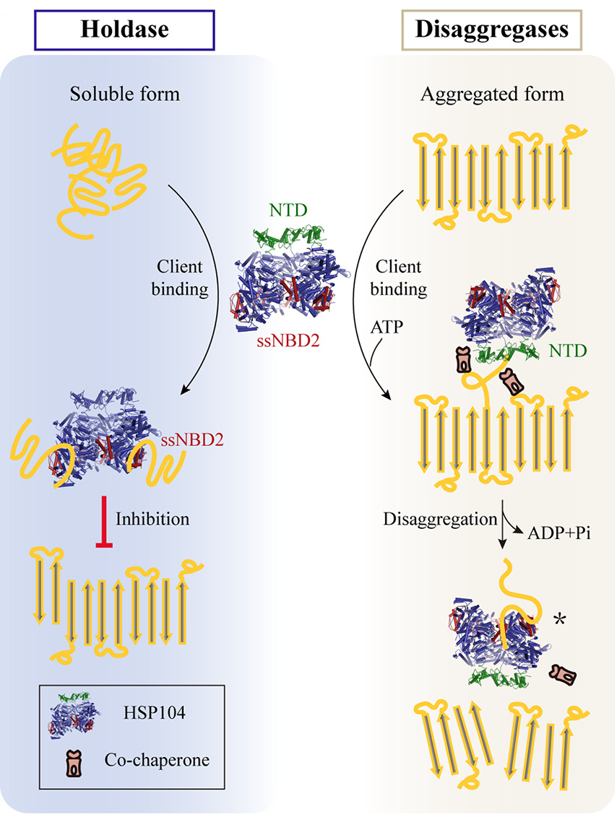
the two distinct chaperoneing activities of HSP104 (Imaged by LIU Cong )
Liu and his colleagues firstly determined the structure of hexameric Hsp104 induced by ATP. Moreover, they revealed that Hsp104 can utilize its NBD2 to capture pathological amyloid proteins Tau and α-Syn which are the key pathological entities in AD and PD, and prevent them from amyloid aggregation. These findings indicate that Hsp104 employs distinct strategies to tackle different forms of amyloid clients, and also highlight the important yet previously un-identified function of ssNBD2 in chaperoning amyloid client and thereby preventing pathological aggregation. This should shed lights on the development of inhibitors for potential therapeutics of amyloid-related diseases.
Author:LIU Cong Shanghai Institute of Organic Chemistry, CAS E-mail: Liulab@sioc.ac.cn |


