Homochirality is a fundamental feature of all living organism. It is well known that a pair of enantiomers of chiral drug molecules, whose structures have a non-superimposable mirror-image relationship to each other, will probably exert different, or even opposite influences in pharmacological activity, metabolism and toxicity. Therefore, the selective synthesis of a pair of enantiomers of given chiral molecules not only is a prominent task in asymmetric synthesis, but also has significant impacts in pharmaceutical chemistry and materials science. In general, the preparation of each enantiomer of chiral target molecules requires the reversal of the absolute configuration of certain chiral component in the reaction system, in any form of chiral substrate, auxiliary, reagent, catalyst or ligand. However, many important chiral source molecules only exist in one configuration in nature. Therefore, the preparation of both enantiomers of chiral target molecules via asymmetric synthesis enabled by such chiral sources are quite challenging.
The research group led by Prof. Shu-Li You at the State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry recently disclosed a unique phenomenon in asymmetric catalysis where the reaction time is found as the controlling factor to determine the absolute configuration of the chiral products. More specifically, in the Ir-catalyzed asymmetric allylic amination reactions, the same chiral Ir-complex enables four independent reaction pathways corresponding to the generation and consumption of the (R) and (S) enantiomers of the target allylic amination products. Thus, the appropriate permutation of the four individual reaction rates guarantees the isolation of each enantiomer of the allylic amination products in a highly enantioenriched form at different reaction time.
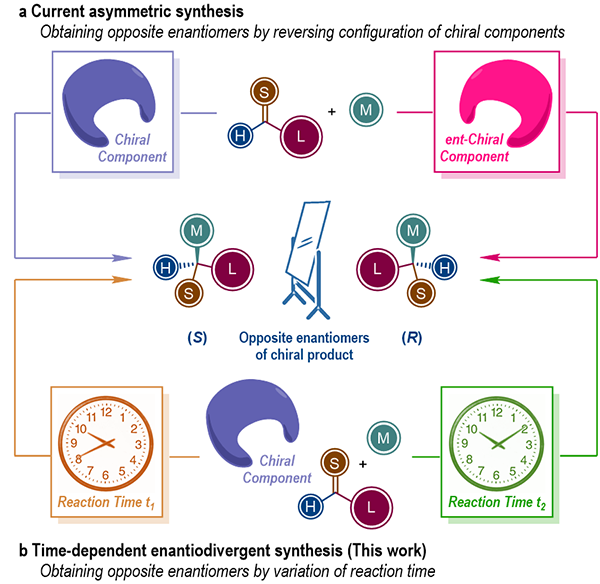
Figure 1. Strategies for obtaining both enantiomers of chiral molecules.
The course of time-dependent accesses to both enantiomers can be illustrated with the story of The hare and the tortoise. The formation of (S) product is just like the hare who is running fast and approaching the (S) product in a very short time. However, the hare is lazy, abandoning the competition very near to the finishing line. One the other hand, although the formation of (R) product, like the tortoise, is rather slow, yet it sticks to running, and finally arrives at the finishing line at a longer time.
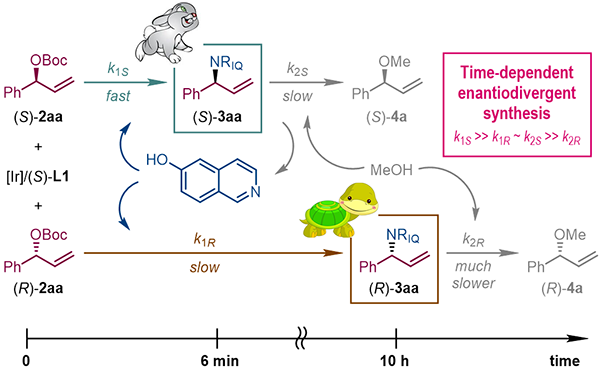
Figure 2. Mechanism of time-dependent accesses to both enantiomers
This finding is unprecedented in asymmetric synthesis. It alerts the organic chemistry community the critical role of reaction time when performing catalytic asymmetric reactions. The research group of Prof. You is currently working on the expansion the scope of this reactivity pattern, and the fast generation of useful chiral molecule libraries for medicinal chemistry.
The results of this study were just published on Nature Chemistry under the title of “Time-dependent enantiodivergent synthesis via sequential kinetic resolution”.(https://www.nature.com/articles/s41557-020-0489-1)
This work is financially supported by National Key R&D Program of China, National Natural Science Foundation of China and Chinese Academy of Sciences.


