Scientists from Shanghai Institute of Organic Chemistry, CAS have recently conducted a systematically biochemical and structural characterization of Syntaxin17 as well as its interactions with the ATG8 family proteins, SNAP29 and VAMP8, and uncovered three different states of Syntaxin17 in mediating autophagosome-lysosome fusion (Figure 1) for the first time.
Autophagy is a highly conserved and lysosome-dependent metabolic process in eukaryotic cells, the dysfunction of which is linked with many human diseases, such as cancer, diabetes and neurodegenerative diseases. In the later stage of autophagy process, double-membraned autophagosomes fuse with single-membraned lysosomes to form autolysosomes, thereby achieving the degradation of autophagosomal inner membrane and enclosed cargoes by lysosomal proteases for recycling.
So far, many proteins have been identified as being involved in the autophagosome-lysosome fusion process, including autophagic SNARE proteins, ATG8 family proteins, tethering factors HOPS complex and ATG14. As a key autophagosomal SNARE protein, Syntaxin17 can associate with ATG8 family proteins, and the other two relevant SNARE proteins, SNAP29 and VAMP8, to facilitate the autophagosome-lysosome membrane fusion process. However, the detailed molecular mechanisms underlying the cooperation of these proteins to promote the formation of the autolysosome are still not well-understood.
In this work, researchers discovered that Syntaxin17 adopts an autoinhibited ‘closed’ conformation in isolation due to an intramolecular interaction between Syntaxin17 SNARE motif and its Habc domain. In addition, they revealed that Syntaxin17 SNARE region contains only one LC3-interaction region (LIR) motif, which preferentially binds to GABARAP subfamily members of the ATG8 family proteins. The determined crystal structure of Syntaxin17 LIR/GABARAP complex further highlights the importance of C-terminal extension following LIR core sequence to selectively interact with ATG8 family proteins. Moreover, they also investigated the biochemical properties of autophagic Syntaxin17/SNAP29/VAMP8 SNARE complex and solved its crystal structure, revealing its conserved biochemical and structural characteristics common to all other SNAREs.
The results were published on the PNAS journal with the title of "Decoding three distinct states of Syntaxin17 SNARE motif in mediating autophagosome-lysosome fusion"
Read the full article here: https://doi.org/10.1073/pnas.2006997117
Pan Lifeng
Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences
Ling Ling Road 345 Shanghai 200032 China
Tel: 0086-21-54925561
Email: panlf@sioc.ac.cn
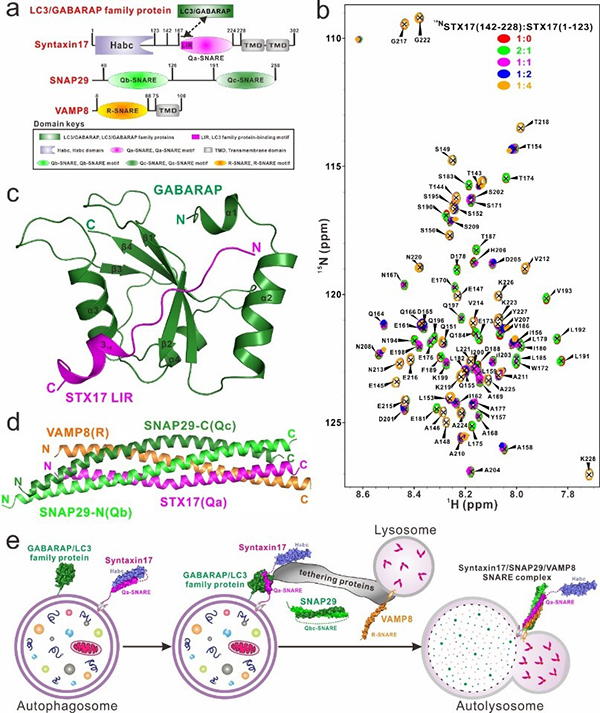
Figure 1. Mechanistic insights into the Syntaxin17-mediated autophagosome-lysosome fusion process


