Z-Olefins widely exist as basic structural motifs in nature. However, Z-olefins are thermody-namically less stable than their E-type counterparts, rendering their efficient and highly selective synthesis a challenging task. It is noteworthy that Z-olefins with a homoallylic stereogenic center are frequently embedded in diverse natural products and bioactive molecules, and have therefore gener-ated considerable interest.
Asymmetric allylic substitution reactions catalyzed by transition-metals, such as Mo, Pd, Ir, and Rh, have been widely used for constructing stereochemically-defined carbon–carbon and carbon–heteroatom bonds at the homoallylic position (Fig. 1). In these reactions, π-allyl-metal complexes in either syn- or anti-configuration are involved as the key intermediates. The relative thermodynami-cally more stable syn-π-allyl-metal complexes dominate the equilibrium between these two types of species via the π-σ-π interconversion process, providing either E-linear or branched olefin products. In this regard, it is quite challenging to synthesize Z-olefins in an enantioselective manner by transi-tion-metal-catalyzed allylic substitution reactions.
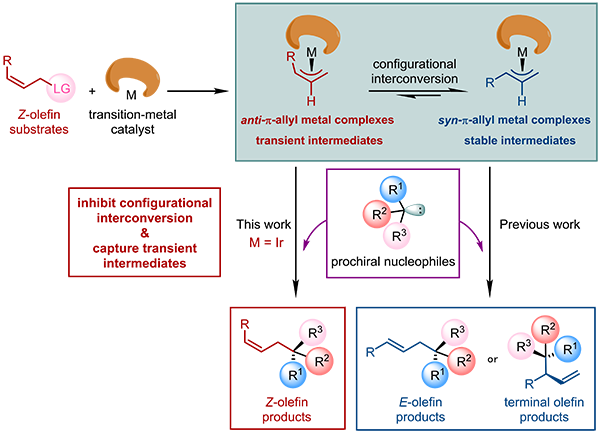
Fig. 1. General mechanism of transition-metal-catalyzed asymmetric allylic substitution reac-tions.
The research group led by Prof. Shu-Li You at the State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry recently proposed a strategy of “capturing tran-sient anti-π-allyl-Ir complexes by reactive external nucleophiles” for the synthesis of complex chiral Z-olefins. This reaction design was successfully executed with an Ir-catalyst derived from a newly synthesized Carreira-type ligand. The proposed reactions between readily available Z-cinnamyl de-rivatives and various indole-derived prochiral nucleophiles afforded Z-cinnamylated pyrroloindolines and analogs in high yields and enantioselectivity. Notably, the Z-type geometry of the olefin moiety is retained exclusively (>20:1 Z/E) in the target molecules (Fig. 2).
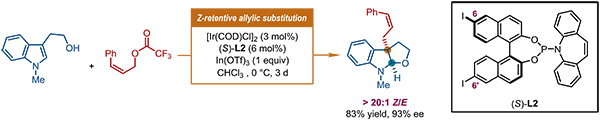
Fig. 2. Ir-catalyzed Z-retentive asymmetric allylic substitution reactions of indole derivatives.
Mechanistic studies including electrospray ionization mass spectrometry (ESI-MS) and nuclear magnetic resonance (31P NMR) provided solid evidence for the formation of anti-π-allyl-Ir complexes, their isomerization to the thermodynamically more stable syn-π-allyl-Ir counterparts and capture by external nucleophiles (Fig. 3). These results demonstrated that the success of the proposed Z-retentive asymmetric allylic substitution reaction originated from the rapid and enantioselective capture of the transient anti-π-allyl-Ir complexes by external nucleophiles prior to their conversion to any other thermodynamically more stable species.
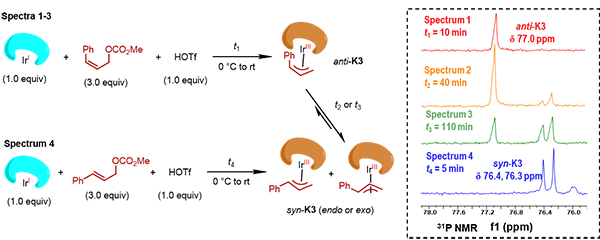
Fig. 3. The formation and isomerization of anti-π-allyl-Ir complexes characterized by ESI-MS and 31P NMR studies.
Z-Retentive asymmetric allylic substitution reaction is found to be general. α-Amino ac-id-derived aldimine esters are also demonstrated to be suitable prochiral nucleophiles. Under modi-fied conditions assisted by a Cu catalyst, Z-retentive asymmetric allylic substitution reaction offered a facile and efficient access to chiral α,α-disubstituted α-amino acid derivatives possessing a Z-olefin moiety (Fig. 4).

Fig. 4. Ir-catalyzed Z-retentive asymmetric allylic substitution reactions of α-amino acid deriva-tives.
The results presented herein reveal an unprecedented reaction mode in transi-tion-metal-catalyzed asymmetric allylic substitution reactions, and can be expected to evolve into a general and practical method for the synthesis of chiral Z-olefins.
The results of this study were just published on Science under the title of “Iridium-catalyzed Z-retentive asymmetric allylic substitution reactions”.
(https://science.sciencemag.org/content/371/6527/380#login-pane)
Shuli You
Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences
Ling Ling Road 345 Shanghai 200032 China
Tel: 0086-21-54925085
Email: slyou@mail.sioc.ac.cn


