Autophagy is a highly regulated and lysosome-dependent intracellular catabolic process in eukaryotes, and is essential for maintaining cellular homeostasis and promoting cell growth, development and survival. The autophagy receptor-mediated selective autophagy can degrade specific autophagic substrates, including misfolded bulk protein aggregates, dysfunctional mitochondria, and invading pathogens. Selective autophagy plays crucial roles in numerous physiological processes, and its dysfunction is associated with a large number of human diseases, including neurodegenerative diseases and microbial infections. In mammalian cells, the ULK complex, which is composed of ULK1, ATG13, ATG101, and FIP200 four subunits, is directly involved in the initiation of phagophore formation. In some selective autophagy processes, autophagy receptors can directly recruit ULK complex by interacting with the FIP200 subunit, and then promote the formation of phagophore in situ near the targeting substrates. However, the detailed mechanism underlying the specific interactions between autophagy receptors and the FIP200 subunit as well as the related regulatory mechanism are still largely unknown.
Recently, a research paper titled “Phosphorylation regulates the binding of autophagy receptors to FIP200 Claw domain for selective autophagy initiation” was published on the Nature Communications journal by Professor Lifeng Pan’s group from Shanghai Institute of Organic Chemistry, CAS (https://www.nature.com/articles/s41467-021-21874-1). In this paper, using multiple biochemical techniques, such as solution NMR, fast protein liquid chromatography, analytical ultracentrifugation and fluorescence polarization, they discovered that the phosphorylation of CCPG1 FIR2 as well as the TBK1-mediated phosphorylation of Optineurin LIR can regulate and enhance the interactions of FIP200 Claw with CCPG1 FIR2 and Optineurin LIR. Subsequently, they determined the high-resolution crystal structures of FIP200 Claw in complex with the phosphorylated CCPG1 FIR2 (p-CCPG1 FIR2) or the phosphorylated Optineurin LIR (p-Optineurin LIR) using X-ray diffraction method for the first time. Consistent with biochemical results, detailed structure analyses revealed that FIP200 Claw forms a stable dimer and symmetrically interacts with two p-CCPG1 FIR2 or p-Optineurin LIR molecules to form a heterotetramer, and the phosphorylation modifications of CCPG1 FIR2 and Optineurin LIR have different regulative mechanism to promote their interactions with FIP200 Claw. Finally, based on their biochemical and structural results, they defined the consensus FIR sequence for the first time, and demonstrated that CCPG1 FIR2 or Optineurin LIR can bind to FIP200 and ATG8 family proteins competitively through the same interface residues. These results suggested that after recognization of substrate, autophagy receptor should firstly recruit the FIP200-containing ULK complex, and then in situ initiate autophagosome formation, and ultimately promote the expansion and closure of the preautophagosomal membrane around the autophagic cargo by interacting with ATG8 family proteins. In summary, their findings uncover a general and phosphoregulatable binding mode shared by autophagy receptors to interact with FIP200 Claw domain for the recruitment of autophay-initiating ULK complex, and are valuable for further understanding the molecular mechanism of autophagy receptor-mediated selective autophagy processes.
The PhD student Zixuan Zhou from Professor Pan’s group is the first author of this paper. This work was supported by grants from the National Key R&D Program of China (2016YFA0501903), National Natural Science Foundation of China (31470749, 21621002, 91753113, 21822705), Science and Technology Commission of Shanghai Municipality (17JC1405200), Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20000000), and the start-up fund from the State Key Laboratory of Bioorganic and Natural Products Chemistry and Chinese Academy of Sciences.
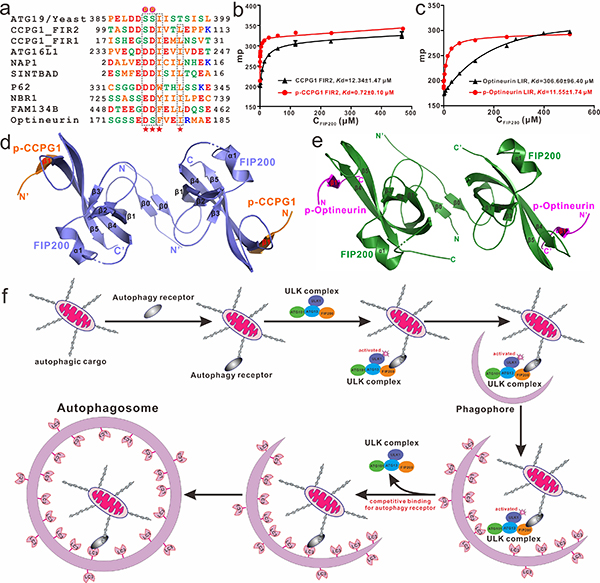
Figure 1. Mechanistic insights into the recruitment of ULK complex by autophagy receptor CCPG1 and Optineurin by binding to FIP200


