Parkinson’s disease (PD) is the second most common neurodegenerative diseases, which is characterized by the accumulation of α-synuclein (α-syn) depositions in Lewy bodies and neurites. The spread of pathological α-syn within the brain and from other organs to the brain is a crucial event in the progression of PD. Cell surface receptors such as lymphocyte activation gene 3 (LAG3) and amyloid precursor like protein 1 (APLP1) can preferentially bind α-syn in the amyloid over monomeric state to initiate cell-to-cell transmission. However, the molecular mechanism underlying this selective binding is unknown. Nor do we know the role of posttranslational modification of α-syn in this progress, e.g., S129 phosphorylation, a hallmark modification of α-syn accumulation in Lewy bodies. On June 21th, 2021, a research paper titled “Mechanistic basis for receptor-mediated pathological α-synuclein fibril cell-to-cell transmission in Parkinson’s disease” was published on the Proceedings of the National Academy of Sciences of the United States of America journal by Prof. Cong Liu from the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, collaborated with Prof. Yan-Mei Li from Tsinghua University, and Prof. Xiaobo Mao and Ted M. Dawson from Johns Hopkins University School of Medicine. In this paper, researchers performed multiple biophysical, cellular, and in vivo approaches to reveal the structural basis underlying the receptor binding of α-syn amyloid fibrils during cell-to-cell transmission (Fig. 1). α-Syn uses its acidic C-terminus to bind an alkaline patch on the cell surface receptors. The formation of amyloid fibrils dramatically enhances the binding by condensing α-syn molecules and better exposing the C-terminus. Moreover, S129 phosphorylation, a pathological biomarker in PD, further strengthens the electrostatic interactions between α-syn and the receptors, which accelerates the PD-like pathology in mice. This work provides the structural basis for the receptor mediated neuronal internalization and transmission of α-syn fibrils and suggests that the C-terminus, specifically residues 118 to 140, is a pathological epitope of α-syn for receptor binding and thus may serve as a promising target for the therapeutic drug development to block PD progression. 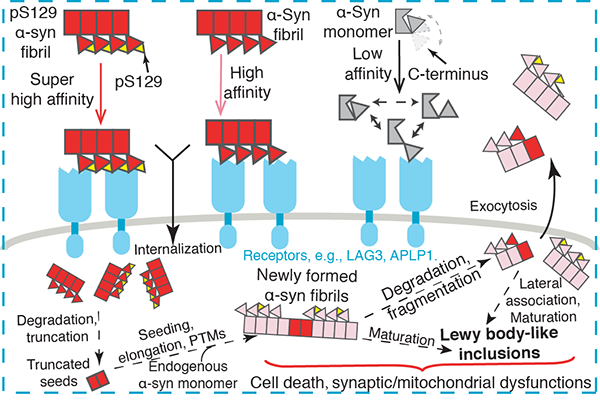 Fig.1 Schematic diagram of α-syn pathological transmission in PD. The co-corresponding authors are Prof. Cong Liu from the Interdisciplinary Research Center on Biology and Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Prof. Yan-Mei Li from Tsinghua University, Prof. Xiaobo Mao and Prof. Ted M. Dawson from Johns Hopkins University School of Medicine. Shengnan Zhang, Yu-Qing Liu, Chunyu Jia, Yeh-Jun Lim, and Guoqin Feng are the co-first authors. This work was supported by National Natural Science Foundation of China, the National Key Research and Development Program of China, the Science and Technology Commission of Shanghai Municipality and so on. Liu Cong Ph.D. Professor Tel: 021-68582528 Email: liulab@sioc.ac.cn |


