Neurodegenerative diseases, such as Alzheimer's disease and Parkinson's disease, are closely associated with pathological aggregation of amyloid proteins in the brain. Amyloid fibrils are regarded as the key clinicopathological markers of these diseases, and are important targets for early diagnosis and therapy of neurodegenerative diseases. Clinical use urgently needs specific small molecular tracers. However, the structures of pathological aggregates exhibit unique properties, which are distinct from native proteins. Current vast knowledge on protein-ligand interaction is based on native proteins, which have been indicated not applicable for protein-ligand interaction in the amyloid state. The underlying mechanism of how small molecules recognize amyloid fibrils is still lacked, which greatly hampers the development and optimization of chemicals that specifically bind pathological aggregates.On July 3, 2023, a research article titled “Structural mechanism for specific binding of chemical compounds to amyloid fibrils” (https://www.nature.com/articles/s41589-023-01370-x) was published on Nature Chemical Biology by LIU Cong group from Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences and LI Dan group from Shanghai Jiao Tong University. In this paper, the researchers systematically uncover the mechanism of ligand binding to amyloid fibrils. This work not only significantly renovates classical theory of protein-ligand interaction in the amyloid state, but also makes it possible to develop small molecule tracers for diagnosing neurodegenerative diseases based on structure.
In this study, the researchers find that compounds with various scaffolds can bind to multiple binding sites of α-synuclein (α-syn) fibrils (histological hallmarks in Parkinson's disease) through three different orientations (Fig. 1a, 1b): diagonal geometry, horizontal geometry, and vertical geometry. The ligand-binding sites of amyloid fibrils are confined in only two dimensions, while is open on the fibril growing axis, therefore inter-ligand π-π stacking needs to be established for specific binding. Both diagonal and horizontal geometries facilitate inter-ligand interactions, though the diagonal geometry generally provides more specificity (Fig. 1c). Thus, in addition to classic ligand-protein interactions, ligand-ligand interactions synergistically contribute to the specificity of small molecules binding on amyloid fibrils. Besides, the researchers identified a druggable pocket conserved in both in vitro and ex vivo α-syn fibrils from patients with multiple system atrophy. The finding of such a pocket will enable screening, rational design and optimization of specific tracers based on this pocket.
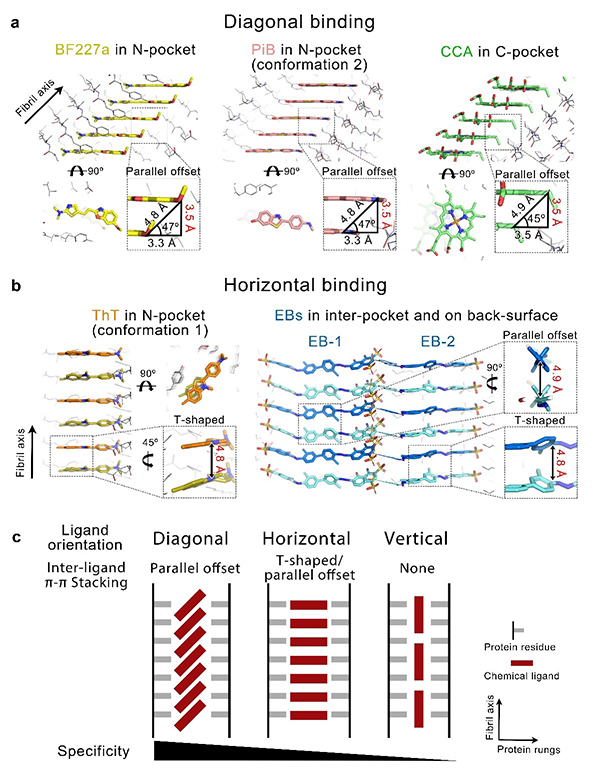
Fig. 1 Binding geometries and specificities in ligand-bound amyloid fibrils(Image by LIU Chong)
Together, this study builds the fundamental knowledge for small molecule ligand-protein interactions in the amyloid state for the first time, and lays foundation for the structure-based development of tracers that can specifically recognize amyloid aggregates. On the basis of these principles, LIU Cong's group is identifying a series of specific lead compounds to image α-syn deposits in Parkinson's disease and other synucleinopathies based on atomic structures and high-throughput screening methods. The technical platform will largely facilitate the related investigation for the design and examination of clinically benefited positron emission tomography (PET) imaging tracers, which will promote the early diagnosis and precise typing of complicated neurodegenerative disorders.
LIU Chong Ph.D.Professor
Interdisciplinary Research Center on Biology and Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences
Haike Road 100 Shanghai 201210 China
Tel: 0086-21-68582528
Email: liulab@sioc.ac.cn


