Hydrogen atom transfer (HAT) process is a powerful and straightforward strategy to realize alkene difunctionalization through a radical reaction pathway from abundant feedstock compounds without pre-functionalization. Thus, applying haloalkyl substrates to this strategy is an effective method to introduce halogen atoms into complex molecules and realize the further transformations to the other important functional groups. However, the high bond dissociation energy (BDE) of non-activated C(sp3)-H bond and the electron-withdrawing effect of halogen atom bring challenge for the HAT process under mild conditions. In this scenario, we developed a photocatalytic HAT process to realize alkene difunctionalization by hydrogen transfer radical addition and radical polar crossover process to afford an array of haloalkane products with broad substrate scope under mild conditions. These findings set up a stage for the application of photocatalytic HAT process in alkene difunctionalizations.Recently, the SHI’s group developed a mild photocatalytic method to generate tert-butoxy radical species which can effectively achieve C(sp3)-H abstraction at halogen’s α-site. Thus, the difunctionalization of aromatic alkenes could be realized by hydrogen transfer radical addition (HTRA) and radical polar crossover (RPC) process, affording an array of haloalkane products with broad substrate scope and excellent functional group tolerance. Moreover, the transformations of haloalkanes to alkanes, haloalkenes or heterocyclic compounds can be accomplished upon simple manipulation. The reaction mechanistic paradigm of the generated tert-butoxy radical species is supported by control experiments and Stern-Volmer analysis, the radical polar crossover is analyzed by Hammett plotting and DFT calculation. The hydrogen atom transfer (HAT) on the C(sp3)-H bond of halogen’s α-site induced by tert-butoxy radical species is also revealed by DFT calculations. 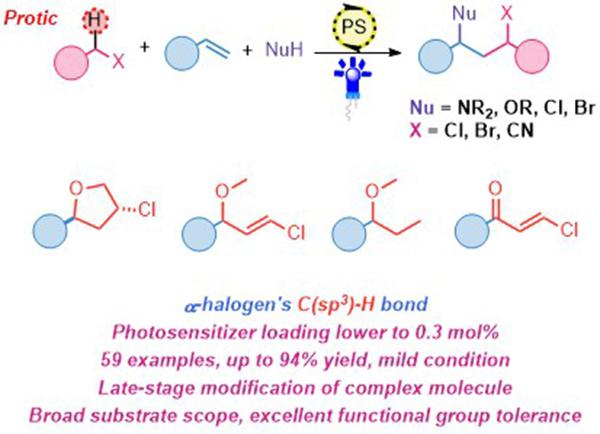
Figure 1. Photocatalytic Alkene Difunctionalization Enabled by Hydrogen Atom Transfer from Haloalkane (Image by SHI Min) The research outcome has been reported on (Chem Catalysis 2023, 10.1016/j.checat.2023.100807) and this work was financially supported by National Natural Science Foundation of China, SIOC and State Key Laboratory of Organometallic Chemistry. SHI Min Ph.D., Professor Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences Ling Ling Road 345 Shanghai 200032 China Tel: 0086-21-54925137 Email:mshi@mail.sioc.ac.cn |


