Z-olefins are frequently found in natural products and pharmaceutical molecules. However, the thermodynamic instability of Z-olefins compared to E-olefins leads to limitations in their highly selective synthesis. The Tsuji-Trost reaction appears to be one of the most efficient techniques to obtain functionalized olefin products. However, the construction of Z-olefins remains a formidable challenge for the Tsuji-Trost reaction due to the thermodynamic instability of anti-π-allyl-Pd complexes generated from the oxidative addition of Pd-catalysts with Z-linear allylic substrates. The anti-π-allyl-Pd complex is well-known to undergo rapid π-σ-π interconversion resulting in the syn-π-allyl-Pd complex. The nucleophilic attack of the latter proceeded, generally leading to the E-olefin products. To date, there have been only sporadic reports that could stabilize the anti-π-allyl-Pd complex. However, the access to Z-olefin will be further hampered by the branched regioselectivity, where the nucleophilic attack towards the more substituted π-allyl terminus leads to the terminal alkene products. Thus, these two factors pose serious challenges for developing the Z-retentive Tsuji-Trost reaction.
The YOU Shu-Li group of the State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, has been focusing on the study of transition metal catalyzed asymmetric allylic substitution reactions. In the previous research, they successfully achieved iridium-catalyzed Z-retentive asymmetric allylic substitution reactions that take advantage of the relatively slow isomerization rate of the π-allyl-Ir complex (Science 2021, 371, 380; J. Am. Chem. Soc. 2022, 144, 4770; Nat. Catal. 2022, 5, 1089.). Given the much broader application of the Tsuji-Trost reaction, the Pd-catalyzed Z-retentive allylic substitution reaction would be highly desirable.
Recently, the You group developed Pd-catalyzed Z-retentive allylic substitution reaction for the first time. The key to success lies in the utilization of a sterically bulky phosphoramidite ligand, which enables the nucleophilic attack step to proceed prior to the π-σ-π isomerization process of the π-allyl-Pd intermediate. Preliminary mechanistic investigations suggest that the sterically bulky phosphoramidite ligand can slow down the π-σ-π isomerization process of the anti-π-allyl-Pd intermediate. In terms of the range of reaction substrates, the synthetic method is broadly applicable to both nucleophiles and Z-allylic carbonates, where N-, S-, C-, and O-nucleophiles all resulted in good Z/E ratios and yields, and trisubstituted Z-allyl carbonates were tolerated in the reaction. In addition, an enantioselective variant has been demonstrated for the synthesis of highly diastereo- and enantioenriched cyclic ketones possessing a Z-alkene moiety starting from Z-allylic carbonates by combing Pd-catalyzed Z-retentive allylic substitution with Cu-catalyzed asymmetric dialkylzinc conjugate addition to cyclic enones. The reaction proceeded smoothly to deliver satisfactory results (32 examples, up to 91% yield, 98% ee).
The research outcome has been reported on (Chem. 2024, http://doi.org/10.1016/j.chempr.2024.02.006) and this work was financially supported by China Postdoctoral Science Foundation, National Key R&D Program of China, National Natural Science Foundation of China, New Cornerstone Science Foundation, Initiative Postdocs Supporting Program and Shanghai Post-doctoral Excellent Program.
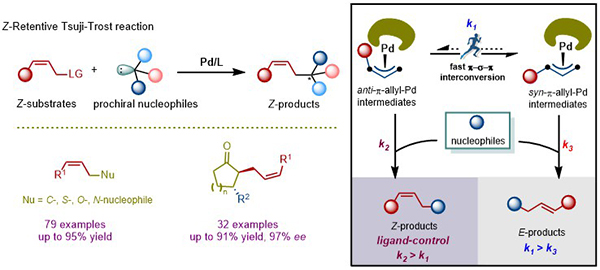
Figure 1. Palladium-catalyzed Z-retentive allylic substitution reactions (Image by YOU Shu-Li)
YOU Shu-Li Ph.D. Professor
Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences
Ling Ling Road 345 Shanghai 200032 China
Tel: 0086-21-5492085
Email: slyou@sioc.ac.cn


