Cells adeptly navigate intricate environments, perceiving and responding to various stimuli, with mechanical signals playing a crucial role in processes like touch and hearing. Mechanosensitive ion channels, a specialized class of proteins, including the OSCA/TMEM63 family, are key players in detecting mechanical cues. When activated by force in the surrounding membrane, these channels transition from a closed to an open conformation, creating a pathway for ions and converting mechanical stimuli into electrochemical signals for downstream signal transduction. Despite their significance, the precise structural basis of how these channels are activated by mechanical force remains largely unknown. One primary obstacle is the difficulty in applying a defined mechanical force during structural data collection, making it a crucial yet challenging area of investigation.In this study, Prof. ZHANG Yixiao’s team aimed to capture the open structure of OSCA/TMEM63 channels using distinct and complementary strategies. Their initial approaches involved nanodiscs, small lipid bilayer patches encircled by a scaffold protein, to mimic increased membrane tension. By reducing the number of lipids per nanodisc, they induced lipid expansion in nanodiscs, thus heightening tension around embedded OSCA/TMEM63 proteins. While these attempts provided hints towards the gating process of these channels, it was structures of OSCA channels reconstituted into small liposomes—spherical lipid vesicles—introducing significant local stress around the channel that gave them the clearest picture of the open OSCA channel. In parallel they utilized computational and electrophysiological approaches to guide and interpret these structural studies. Through these combined structural, functional and computational studies they revealed how the OSCA/TMEM63 channels react to mechanical force, with lipids playing distinct and critical roles in the conformational transitions. The most exciting observation was that, unlike typical ion channels where the pore is completely surrounded by protein, the pore in their activated OSCA channel exhibited a lateral opening to the membrane—meaning the pathway for ion permeation was lined by lipids. Their structural and simulation data showed that headgroups of lipids organize along the pore, acting as a lipid wall to guide ion passage (Fig. 1). This configuration is most strongly supported by their functional data showing that lipid headgroup charge can modulate the ion selectivity of the channel. Thus, their work not only unveils a global view of the gating cycle of OSCA/TMEM63 channels but also identifies unequivocally a 'proteo-lipidic' pore for ion permeation, first proposed by Criss Hartzell in TMEM16 proteins. Given that the wall lipids line the pore, and considering the related TMEM16 proteins' ability to scramble lipids, one might immediately question whether OSCA/TMEM63 channels can function as mechanosensitive lipid scramblases, and what could be the physiological role of such scramblase activity. In future studies, Prof. ZHANG Yixiao’s team are excited to explore the potential mechanosensitive lipid scramblase properties of OSCA/TMEM63 channels and apply their force-mimicking methods to other mechanosensitive channels such as PIEZOs and TMCs. 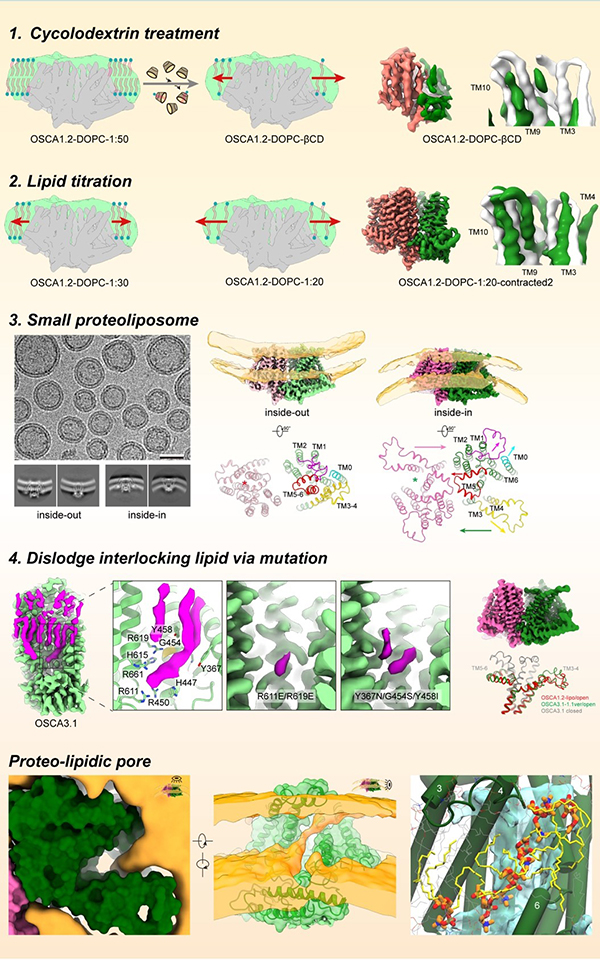
Fig.1 The proteo-lipidic pore of OSCA1.2 (image by ZHANG Yixiao) ZHANG Yixiao Ph.D.Professor Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences Ling Ling Road 345 Shanghai 200032 China Tel: 0086-21-68582391 Email: yzhang@sioc.ac.cn |


