Given that alcohol is the most frequent functional group found in bioactive natural products and marketed drugs, and is versatile synthetic intermediate with a middle level of carbon oxidation state with massive synthetic applications, accessing alcohol-containing architectures via difunctionalization of alkenyl alcohols is highly fascinating. In addition to well-known challenges for dicarbofunctionalization of unactivated alkenes, the main obstacles to develop the difunctionalization reaction with alcohol as the native directing group are their low binding affinity for transition metals, and their conformational flexibility relatively to more commonly used native functional group, such as acid and amide. In a recently study published in Chem Catal. Prof. WANG Peng and coworkers at Shanghai Institute of Organic Chemistry of the Chinese Academy of Sciences disclosed a Ni(II)-catalyzed 1,2-arylalkylation and 1,2-alkenylalkylation of alkenyl alcohols in a regioselective manner with alcohol group as the native directing group, enabled by a bulky β-diketone ligand. This methodology opens a new avenue for the modular synthesis of biologically active compounds and alcohol-containing synthetic intermediates. 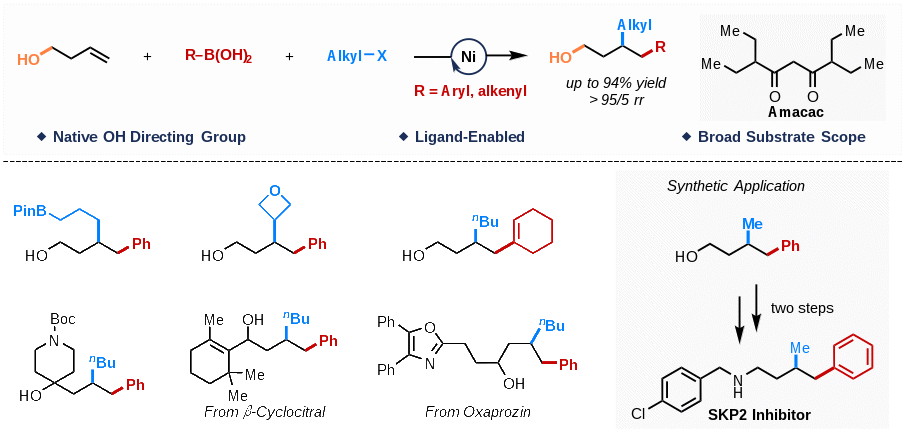
Fig. 1 Ligand-Enabled Ni-Catalyzed Dicarbofunctionalization of Alkenyl Alcohols (Image by WANG Peng) Researchers found that bulky β-diketone ligand (Amacac) plays a crucial role in enhancing the reactivity and suppressing many competitive processes. The obtained complex of Ni2(Amacac)4(EtOH)2 shows high catalytic reactivity (88% NMR yield), which indicates that the β-diketone coordinated nickel complex is probably the active catalyst precursor. “This bulky β-diketone ligand could act as either an LL type or LX type of ligand, depending on the chemical environment of Ni center, which offers a means to fine-tune the Lewis acidity of catalyst, enhancing the reactivity of the catalyst in several elementary steps.” explained by Prof. WANG. 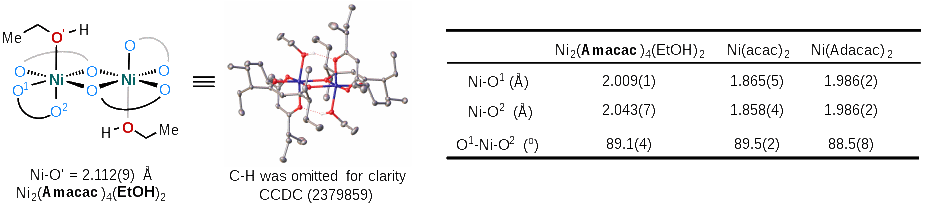
Fig 2. Structural Properties of Ni(Amacac)2 (Image by WANG Peng) This protocol accommodates a wide range of substrates with excellent functional group tolerance, paving the way for the efficient preparation of alcohol-containing synthetic intermediates and bioactive compounds.
WANG Peng Ph.D. Professor Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences Lingling Road 345 Shanghai 200032 China Tel: 0086-21-54925554 Email: pengwang@sioc.ac.cn |


