Rab7A, a member of the Rab family of small GTPases, plays a crucial role in autophagy and endosome pathways in mammalian cells. The activation of Rab7A from its GDP-bound state to GTP-bound state is a key step for the regulation of the activity of Rab7A, and is mediated by its cognate guanine nucleotide exchange factor (GEF), the MON1A/CCZ1/C18orf8 complex. However, the detailed molecular mechanisms governing the formation of the MON1A/CCZ1/C18orf8 complex and its specific GEF activity for Rab7A remain elusive. Recently, a research paper titled “Mechanistic insights into the GEF activity of the human MON1A/CCZ1/C18orf8 complex” was published on the Protein & Cell journal by Professor PAN Lifeng and Professor ZHANG Yixiao groups from Shanghai Institute of Organic Chemistry, CAS (https://doi.org/10.1093/procel/pwaf018). In this paper, using Cryo-EM method, the researcher team determined, for the first time, the structure of the human MON1A/CCZ1/C18orf8 complex bound to Rab7A, revealing a unique architecture with Rab7A interacting with the LD1 domains of MON1A and CCZ1. The study revealed that the formation of the MON1A/CCZ1/C18orf8/Rab7A complex induces significant conformational changes in the Switch I region of Rab7A, thereby destabilizing the GDP binding and promoting the GTP loading of Rab7A. Particularly, several key residues in Rab7A, such as F33, Y37, and K38, interact with the MON1A/CCZ1/C18orf8 complex to facilitate the release of GDP and the subsequent GTP loading. Meanwhile, the study also highlighted that the GTP-bound form of Rab7A cannot bind to the MON1A/CCZ1/C18orf8 complex, as GTP competes with the complex for binding to the key Y37 residue of Rab7A, thereby ensuring that only the GDP-bound Rab7A is recognized by the MON1A/CCZ1/C18orf8 complex. In all, this study not only elucidates the detailed molecular mechanism underlying the activation of Rab7A mediated by the MON1A/CCZ1/C18orf8 complex, but also advances our understanding of the regulation mechanism of Rab7A. Dr. TANG Yubin from Professor PAN’s group and the PhD student HAN Yaoyao from Professor ZHANG’s group are the co-first authors of this paper. This work was supported by grants from the National Natural Science Foundation of China (32071219, 32071297, 92253301, 21822705), the CAS Youth Interdisciplinary Team (JCTD-2022-10), the National Key R&D Program of China (2022YFA1304700), and the National Basic Research Program of China (2022YFC2303102). 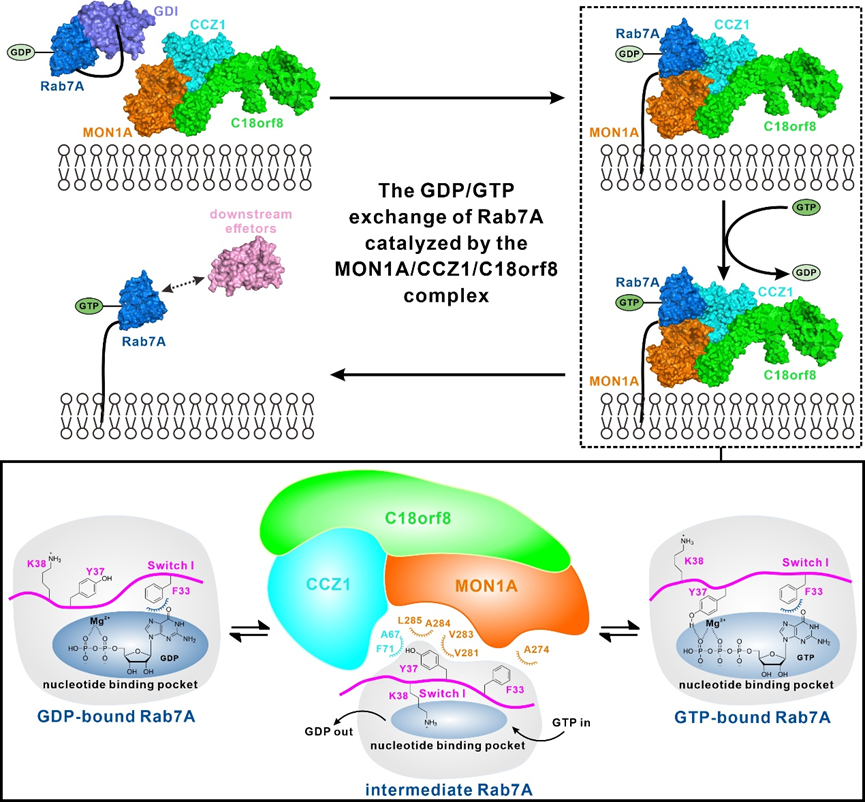
Figure 1. Schematic diagram of the working mode of the MON1A/CCZ1/C18orf8 complex catalyzing Rab7A nucleotide exchange(Image by PAN Lifeng)
PAN Lifeng Ph.D.Professor Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences Ling Ling Road 345 Shanghai 200032 China Tel: 0086-21-54925561 Email: panlf@sioc.ac.cn |


