Subcellular lipid composition and transport significantly influence the physiological and pathological functions of both cells and organelles. However, lipid transport and turnover between organelles remain poorly understood due to a lack of methods for selectively labeling lipids in organelles.
In a study published in Nature Chemistry, research teams led by Prof. ZHU Zheng-Jiang and Prof. CHEN Yiyun at Shanghai Institute of Organic Chemistry (SIOC) of the Chinese Academy of Sciences develop a subcellular photocatalytic labeling strategy that enables the organelle-selective lipid analysis by mass spectrometry and the quantitative profiling of lipid transport between organelles.
Researchers first innovatively developed a proximity labeling strategy for selective lipid analysis. Based on the structural characteristics of lipids, a photocatalytic proximity labeling probe was specifically designed and optimized for lipid reactivity. Organic dyes with organelle-specific localization were used as photocatalysts to activate the probe under blue light irradiation, generating reactive intermediates that selectively label lipids within specific organelles and yielding photocatalytically labeled (PL) lipids. Detection of PL-lipids was achieved through liquid chromatography–mass spectrometry (LC-MS)-based lipidomic analysis, without the need for organelle separation.
Using this technique, researchers identified 60 to 80 lipid species within mitochondria, nucleus, and lysosomes, respectively, encompassing seven major lipid classes: phosphatidylethanolamine (PE), plasmenylethanolamine (PE(P)), lysophosphatidylethanolamine (LPE), lysoplasmenylethanolamine (LPE(P)), phosphatidylserine (PS), sphingosine (Sph), and cholesterol (Chol). A series of biochemical validations—including immunofluorescence colocalization, conventional organelle fractionation, and gene knockout experiments—confirmed the high subcellular specificity of the proximity labeling lipidomics approach, establishing a powerful tool for investigating the spatial distribution of lipids under physiological conditions.
In eukaryotic cells, most phospholipids are de novo synthesized in the ER and then transported to other organelles. To characterize lipid transport between organelles, the research teams developed a dual-labeling strategy by combining subcellular proximity labeling lipidomics with stable isotope tracing. This approach enabled systematic and quantitative characterization of lipid transport from the ER to various organelles, revealing key features such as transport kinetics, turnover, and the relative contributions of different lipid biosynthetic and transport pathways to the lipid composition of specific organelles.
Furthermore, this method uncovered that the lipid transport proteins VPS13A and PDZD8 play crucial and selective roles in mediating ER–mitochondria lipid transfer with fatty acyl chain specificity. In addition, the strategy revealed that mTOR activation selectively increases lysosomal levels of cholesterol, PE, and PS, without altering the global cellular lipidome, providing powerful support for dissecting organelle-specific lipid metabolism.
This work was supported by National Natural Science Foundation of China, the Ministry of Science and Technology of China, the Chinese Academy of Sciences, and the Shanghai Municipal Science and Technology Commission, among others.
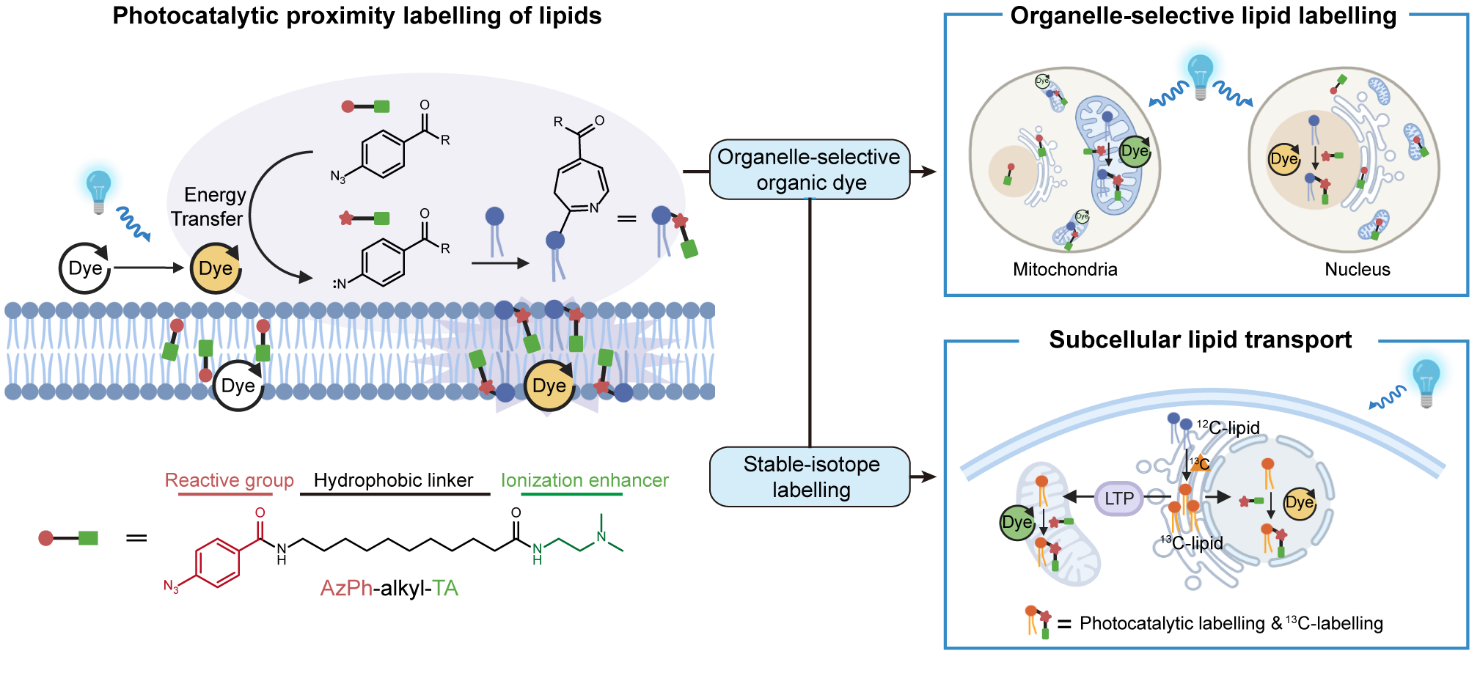
Figure1. Photocatalytic labeling of lipids with organic dyes as photocatalysts. (Image by the SIOC)
ZHU Zheng-Jiang Ph.D. Professor
Interdisciplinary Research Center on Biology and Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences
100 Haike Road, Building 13, Pudong, Shanghai, P. R. China 201210
Tel: 0086-21-68582296
Email: jiangzhu@sioc.ac.cn
CHEN Yiyun Ph.D. Professor
Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences
345 Lingling Road, Shanghai, China, 200032
Tel: 0086-21-54925508
Email: yiyunchen@sioc.ac.cn


