Planar chiral ferrocene derivatives have garnered considerable attention owing to their broad applications in asymmetric catalysis, medicinal chemistry, and materials science. The development of highly efficient approaches for their asymmetric synthesis is therefore of significant interests. Among various strategies, transition-metal-catalyzed asymmetric C–H functionalization has emerged as a powerful and versatile platform. Nevertheless, the reliance on a directing group inherently limits the generality and scope of such methodologies. In a study published in Chem Catalysis on Nov. 20, a research team led by Prof. YOU Shu-Li and Prof. ZHENG Chao at Shanghai Institute of Organic Chemistry (SIOC) of the Chinese Academy of Sciences developed an asymmetric C–H alkenylation of ferrocenes via rational substrate design in combination with a transient directing strategy. Mechanistic experiments, together with DFT calculations, revealed that the size of the palladacycle intermediate generated after C–H cleavage played a decisive role in governing the site-selectivity of the transformation. 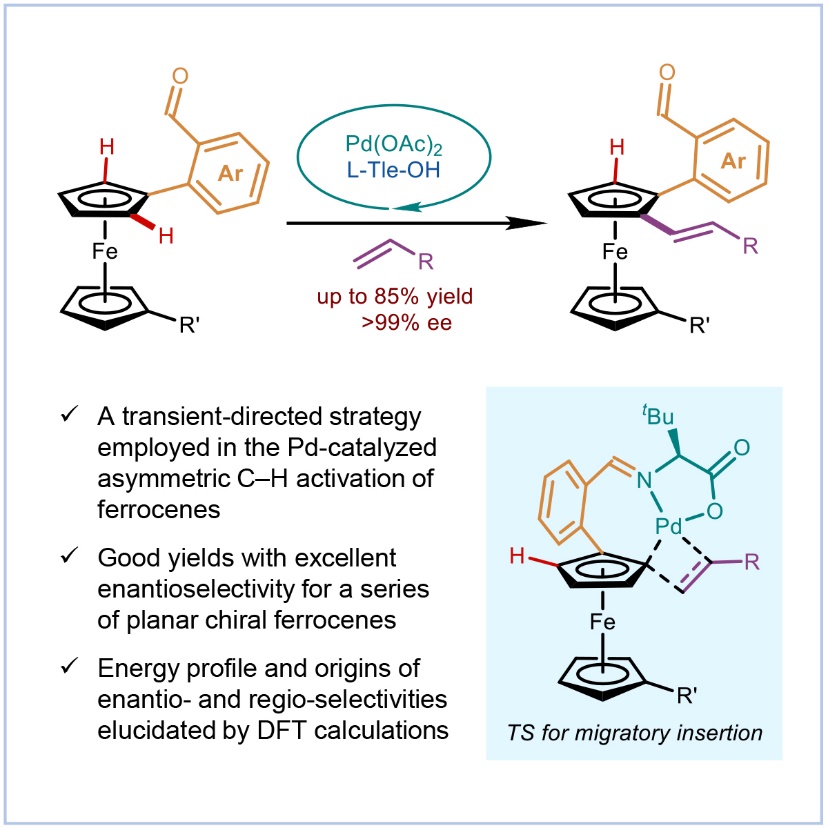
Figure 1. Asymmetric C–H Functionalization of Ferrocenes via Transient Directing Group Strategy (Image by Chem Catalysis) Based on the seminal work of Yu, Shi, and coworkers on the construction of central and axial chiral molecules via transient directing group (TDG) enabled C–H functionalization reaction, the researchers initially envisioned employing ferrocenecarboxaldehyde as the substrate in combination with chiral amino acids as transient chiral auxiliaries. However, according to the work of J. You’s group in 2022, such C–H functionalization preferentially occurs at the 1′-positions of ferrocene rather than at the ortho position adjacent to the aldehyde group. In this regard, the researchers pursued a rational substrate design strategy by introducing a phenyl spacer between the aldehyde functionality and the ferrocene core, thereby using 2-formylphenylferrocene as the substrate. Through systematic optimization of the reaction parameters, an efficient ortho C–H alkenylation of 2-formylphenylferrocene was successfully developed, delivering the desired planar chiral ferrocenes in excellent yields (up to 85% yield) and with outstanding enantioselectivity (up to >99% ee). This strategy not only overcomes the intrinsic regioselectivity challenge associated with direct C–H activation of ferrocenecarboxaldehyde but also broadens the synthetic utility of the TDG approach in planar chiral ferrocene chemistry. Moreover, the aldehyde moiety in 2-formylphenylferrocene serves as a versatile synthetic handle for downstream functional group interconversions. A variety of transformations of the aldehyde functionality can be readily accomplished, enabling access to a broad range of structurally diverse planar chiral ferrocene derivatives. Subsequently, to gain deeper insight into the origin of the regioselectivity observed in the reaction, the researchers performed combined experimental and computational mechanistic studies, which have revealed the importance of storing ring strain in a seven-membered palladacycle after the C–H functionalization. It promotes the downstream migratory insertion with the alkenylation reagents in a strain-releasing manner. The C–H activation at the 1′-position of ferrocene is completely suppressed by the substrate design. In summary, this work offers an efficient and highly selective approach for the synthesis of planar chiral 1,2-disubstituted ferrocenes. Mechanistic investigations combined with DFT calculations elucidate the origin of the regioselectivity, providing a reliable reference for future studies.
Professor YOU Shu-Li Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences 345 Lingling Lu, Shanghai 200032, China Tel: 0086-21-54925085 Email: slyou@sioc.ac.cn |


