As one of the oldest reactions in organometallic chemistry, the Ullmann reaction has a history of over 120 years since its discovery. It is one of the most widely used copper-mediated coupling reactions, extensively applied in the construction of carbon-carbon and carbon-heteroatom bonds due to its excellent substrate generality. However, for a long time, there has been considerable controversy regarding the redox mechanism of copper in this reaction. The currently widely accepted mechanistic hypothesis involves a Cu(I/III) cycle, but copper(III) species are extremely difficult to observe in real reaction systems, and it remains unknown whether other interactions exist between copper species. Recently, SHEN Qilong’ lab from the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, in collaboration with Professor K. N. Houk from the University of California, Los Angeles, provided solid evidences that the Ullmann-type reaction might proceed via a Cu(I)/Cu(III)/Cu(II)/Cu(III)/Cu(I) catalytic cycle. The related findings, titled "Decoding the Redox Behaviour of Copper in Ullmann-Type Coupling Reactions", were published online as an "Accelerated Article Preview" in Nature on September 22, 2025 (https://doi.org/10.1038/s41586-025-09627-2). 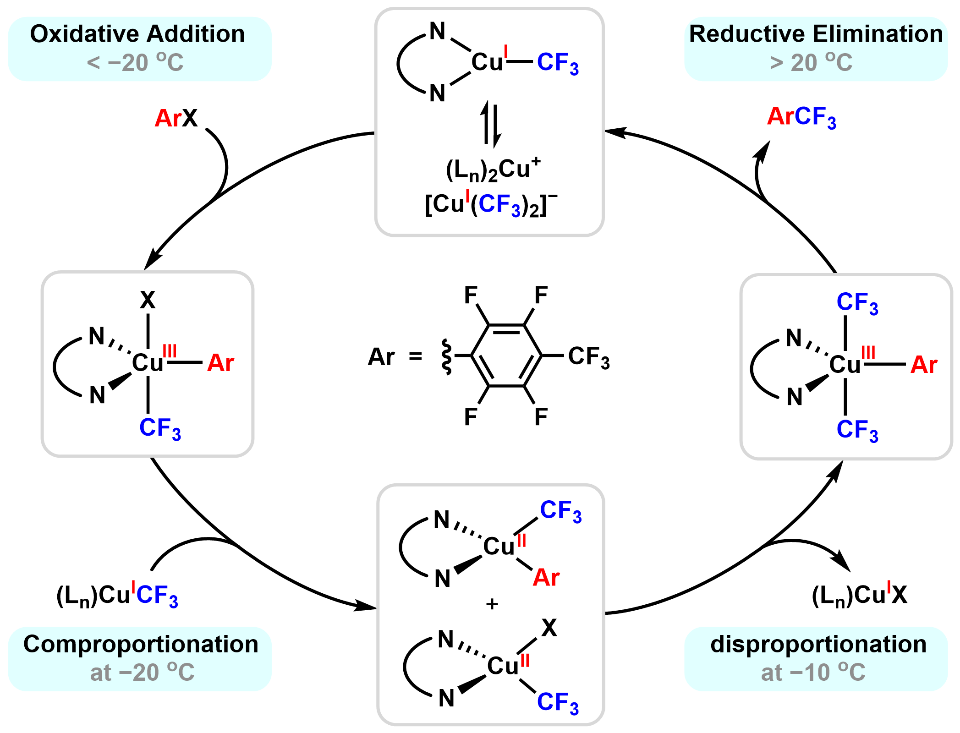
Figure 1. Redox behavior of copper in Ullmann-type trifluoromethylation reactions. (Image by SEHN Qilong’ lab) In the aforementioned study, SHEN 's group uncovered the complex redox behavior of copper species in the reaction by controlling the reaction progress between copper(I) trifluoromethyl complexes and electron-deficient aryl iodides through temperature regulation. The study found that at −20 °C, oxidative addition and comproportionation occur fast, generating copper(II) species. Further increasing the reaction temperature to −10 °C, the Cu(II) species undergo disproportionation to form copper(III) and copper(I) species; finally, the copper(III) species undergo reductive elimination near room temperature, regenerating copper(I) species and completing the entire redox cycle.These processes were cross-validated by multiple spectroscopic techniques, including Nuclear Magnetic Resonance, Electron Paramagnetic Resonance, and Ultraviolet-visible spectroscopy. More importantly, similar reaction behaviors were observed in the trifluoromethylation of various electron-deficient aryl iodides and in Ullmann biphenyl synthesis, suggesting that this mechanism might be a common pathway in Ullmann-type cross-coupling reactions. This study discloses a multi-step cycle involving Cu(I)/Cu(III)/Cu(II)/Cu(III)/Cu(I), indicating that the mechanism of the Ullmann reaction is far more complex than any previously proposed hypothesis, while also providing new mechanistic insights into other copper-catalyzed reactions. This study was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences and the Key Program of the National Natural Science Foundation of China.
SHEN Qilong Ph.D.Professor Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences Lingling Road 345 Shanghai 200032 China Tel: 0086-21-54925197 Email: shenql@mail.sioc.ac.cn |


