NMNAT2 is a key protein required for axon integrity, whose rapid depletion following axon injury triggers Wallerian degeneration. Despite its importance, the molecular mechanism controlling NMNAT2 turnover in neurons has not been fully understood. In a recent study published online in Journal of Cell Biology on September 30, a research team led by Prof. FANG Yanshan from the Shanghai Institute of Organic Chemistry of the Chinese Academy of Sciences was the first to identify FBXO21 as the F-box protein and key factor mediating the ubiquitination and degradation of NMNAT2 in neurons. Knockdown of Fbxo21 in mouse dorsal root ganglion neurons increased NMNAT2 levels and significantly delayed Wallerian degeneration. The field had always believed that NMNAT2 degradation is accelerated in axons upon injury, and that NMNAT2 might have different regulatory mechanisms in neuronal soma and axons. However, in this study, the team demonstrated that the turnover rate of NMNAT2 protein in the soma and axons, as well as before and after injury, is comparable, suggesting a unified mechanism controlling NMNAT2 degradation. The rapid depletion of NMNAT2 in injured axons is mainly due to the lack of the supply of newly synthesized NMNAT2 protein from the soma. Importantly, in both the soma and axon, whether or not injured, FBXO21 regulates NMNAT2 stability and axon integrity. Using denaturing immunoprecipitation and in vitro ubiquitination assays, the team demonstrated that FBXO21 interacts with SKP1, CUL1 and RBX1 to form an SCFFBXO21 E3 ligase complex that mediates NMNAT2 ubiquitination. SCFFBXO21 ubiquitinates NMNAT2 at K155 within the isoform-specific targeting and interaction domain (ISTID). The ubiquitination-deficient mutation K155R significantly prolongs the half-life of NMNAT2 and markedly enhances its axonal protection. Interestingly, swapping the ISTID of NMNAT1 or NMNAT3 with that of NMNAT2 resulted in rapid degradation of the originally very stable proteins, while introducing the K155R mutation eliminated this effect. These results highlight K155 ubiquitination within the ISTID as a key evolutionary determinant of the ultra-short half-life of NMNAT2, revealing the molecular and structural basis of the unique lability of NMNAT2. Finally, the team generated Fbxo21 knockout mice and revealed that NMNAT2 protein levels were specifically elevated in the nervous system. Furthermore, using a mouse sciatic nerve injury model, they demonstrated that Fbxo21 knockout significantly delayed Wallerian degeneration in vivo. In summary, the study uncovered that FBXO21 plays a key role in regulating NMNAT2 stability (Figure 1). Notably, since other components of the SCF complex (such as SKP1, CUL1 and RBX1) are widely involved in degradation of other proteins and not targetable, the discovery of the substrate-determining F-box protein FBXO21 for NMNAT2 is especially significant. It provides a potential target and intervention strategy for enhancing NMNAT2-dependent axon survival in neural injury and degenerative diseases. 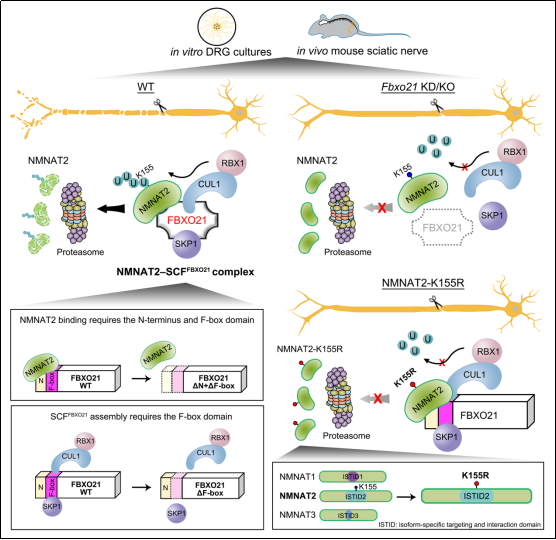
Figure 1. A schematic of the role and mechanism of FBXO21 in regulating NMNAT2 stability and axon survival (Image by LONG Wenjing and FANG Yanshan) FANG Yanshan, Ph.D. Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences Interdisciplinary Research Center on Biology and Chemistry 100 Haike Road, Shanghai 201203,China Tel: 0086-21-68582510 Email: fangys@sioc.ac.cn |


