Metal ions, coordinating with nearly half of the entire proteome, are indispensable players in nearly every biological process, from enzymatic catalysis to signal transduction. Yet, mapping their interactions with proteins across the entire proteome has remained a major technical challenge, limited by sensitivity, specificity, and coverage. A new study published online in Science Advances on October 17, “CysMP reveals metal ion-specific metalloproteomes and copper-regulated PGK1 activity in glycolysis,” led by Prof. ZHANG Yaoyang at the Interdisciplinary Research Center on Biology and Chemistry, Shanghai Institute of Organic Chemistry, CAS, introduces a breakthrough cysteine-centered metalloproteomics strategy named CysMP (Cysteine-centered Metalloproteome Profiling) (Figure 1). This innovative method provides the first comprehensive view of metal-specific proteomes across eleven essential metal ions, marking a significant advance in metalloproteomics. 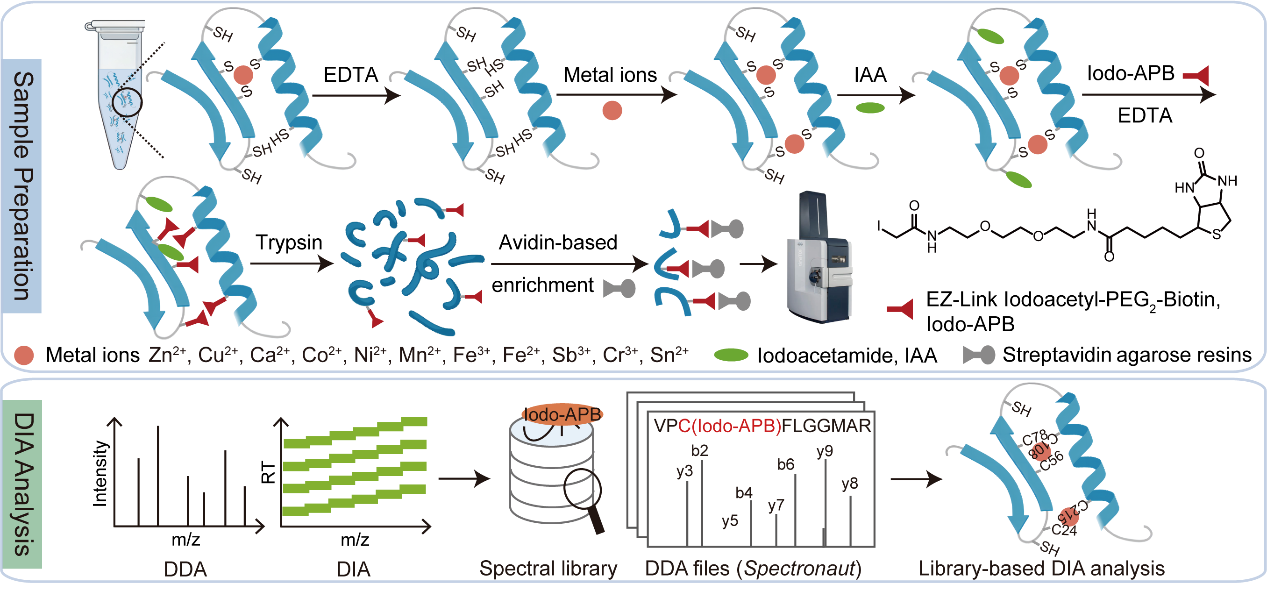
Figure 1. Workflow of the CysMP strategy. (Image by YUAN Yamei and ZHANG Yaoyang) CysMP systematically profiles protein–metal interactions in mammalian cells with unprecedented depth and accuracy, revealing how these essential elements reshape cellular proteome. The study identified 8,895 metal-binding cysteine sites across 4,150 proteins—the most comprehensive cysteine-centered metalloprotein dataset reported to date—enabling quantitative comparisons among different metals and uncovering both binding promiscuity and selectivity. Among the discoveries, zinc and copper ions exhibited the broadest range of protein interactions, revealing unexpected metalloproteins involved in key biological pathways. Notably, CysMP identified copper and zinc binding to the enzyme 5′-methylthioadenosine phosphorylase (MTAP), which inhibited its enzymatic activity and led to MTA accumulation—a phenomenon that could have profound effects on cellular metabolism. Even more strikingly, copper ions were found to suppress the activity of phosphoglycerate kinase 1 (PGK1) (Figure 2), a central enzyme in glycolysis, thereby downregulating glycolysis-related metabolites and reducing cell viability. The copper-dependent regulation of PGK1 revealed by CysMP also provides new molecular insights into the recently emerging concept of cuproptosis—a copper-induced cell death pathway—highlighting how metal ion homeostasis is tightly coupled to metabolic control and cell fate decisions. 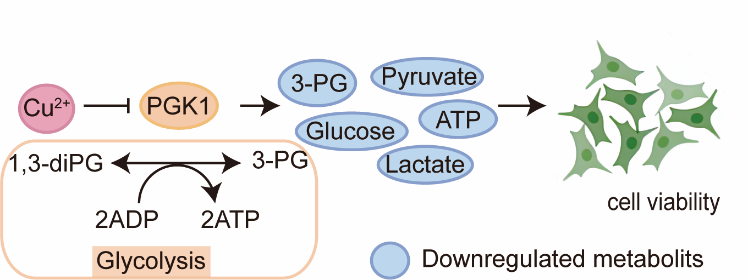
Figure 2. Copper ions inhibit PGK1 activity, leading to reduced glycolytic activity and suppressed cell viability. (Image by YUAN Yameiand ZHANG Yaoyang) The CysMP-generated dataset provides a transformative resource for exploring how metal ion dysregulation contributes to diseases including cancer, metabolic disorders, and neurodegenerative conditions. By linking metal-binding events to enzymatic activity changes, CysMP opens new avenues for therapeutic discovery and precision medicine targeting metal homeostasis. “CysMP provides a powerful, metal-specific window into the metalloproteome,” said Prof. ZHANG. “It not only delivers the most comprehensive resource of metal-binding cysteine sites in mammalian systems, but also redefines how we study the regulatory power of metal ions in life processes.” Overall, CysMP represents the first global profiling of multiple biologically relevant metal ions at site-level resolution. By illuminating this previously hidden regulatory layer, the study sets a new benchmark for metalloproteomic research, revealing the intricate network of metal-mediated control underlying cellular physiology and disease.
ZHANG Yaoyang Ph.D.Professor Interdisciplinary Research Center on Biology and Chemistry (IRCBC) Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences Haike Road 100 Shanghai 201204 China Email: zyy@sioc.ac.cn |


