Autophagy is a highly regulated and critical intracellular catabolic process involving lysosome-dependent degradation of undesired cytosolic components, such as dysfunctional organelles, invasive pathogens, and bulk protein aggregates, for cellular homeostasis and/or adaptation to various stresses in mammals. Autophagy plays an important role in many physiological processes, including cell growth, innate immunity, and aging. Meanwhile, abnormal function of autophagy is closely related to many human diseases such as cancer and neurodegenerative diseases. During the canonical amino acid starvation-induced macroautophagy process, cell relies on autophagosome by triggering and extending the pre-autophagosomal structure (PAS) to encapsulate the relevant autophagy substrate to be degraded in the cell. During PAS initiation and extension, both WIPI2 and FIP200 can recruit the ATG12-ATG5-ATG16L1 complex (ATG16L1 complex) to PAS through binding to ATG16L1, thereby enabling the PE-lipid modification of ATG8 family proteins. Although the mechanism by which WIPI2 recruits and activates the ATG16L1 complex is well understood, the specific molecular mechanism by which FIP200 mediates the recruitment of the ATG16L1 complex to the PAS remains unclear. Recently, a research paper titled “Molecular bases of the interactions of ATG16L1 with FIP200 and ATG8 family proteins” was published on the Nature Communications journal by Professor PAN Lifeng’s group from Shanghai Institute of Organic Chemistry, CAS. In this paper, using various biochemical techniques, such as fluorescence polarization experiment, isothermal titration calorimetry (ITC), multi-angle light scattering (MALS) and liquid Nuclear Magnetic Resonance (NMR), they discovered that ATG16L1 contains a FIP200-interacting region (FIR), which not only can directly bind FIP200 Claw domain, but also can serve as an atypical ATG8-interacting motif to selectively recognize mammalian ATG8 family proteins (ATG8s). Through the determination of the high-resolution crystal structures of ATG16L1 FIR in complex with FIP200 Claw and the ATG8 family member GABARAPL1, respectively, they elucidated the molecular mechanism governing the interactions of ATG16L1 with FIP200 and ATG8s. At the same time, their biochemical results demonstrated that ATG8s can compete with FIP200 for binding to ATG16L1, while WIPI2 and FIP200 can simultaneously bind to ATG16L1. To distinguish the precise contribution of FIP200 from ATG8s for binding to ATG16L1 FIR in autophagy, they rationally devised the ATG16L1 DRIF mutant that can exclusively interact with ATG8s but not FIP200 as well as the ATG16L1 IQIQ mutant that binds neither ATG8s nor FIP200. Finally, they utilized the aforementioned ATG16L1 mutants and conducted related cell biology experiments, revealing that the FIP200/ATG16L1 interaction is essential for the normal progression of classical amino acid starvation-induced macroautophagy, while the ATG8s/ATG16L1 interaction inhibits this autophagy process.Based on relevant research findings, they proposed, for the first time, that the interactions between FIP200/ATG16L1 and WIPI2/ATG16L1 enhance the initiation of autophagy through positive feedback loop in amino acid starvation-induced classical autophagy. In summary, this study has conducted comprehensive biochemical and structural characterizations of the binding mechanisms between core autophagy proteins ATG16L1, FIP200, and ATG8 family members. They discovered, for the first time, that ATG16L1 can competitively bind FIP200 and ATG8 family proteins through its FIR region. By resolving relevant complex structures, we revealed the molecular mechanism of ATG16L1 for binding to FIP200 and ATG8 family members. Furthermore, relevant cellular functional experiments confirmed that the FIP200/ATG16L1 interaction plays an indispensable role in classical autophagy. These findings lay a good foundation for further understanding the molecular mechanism of macroautophagy. 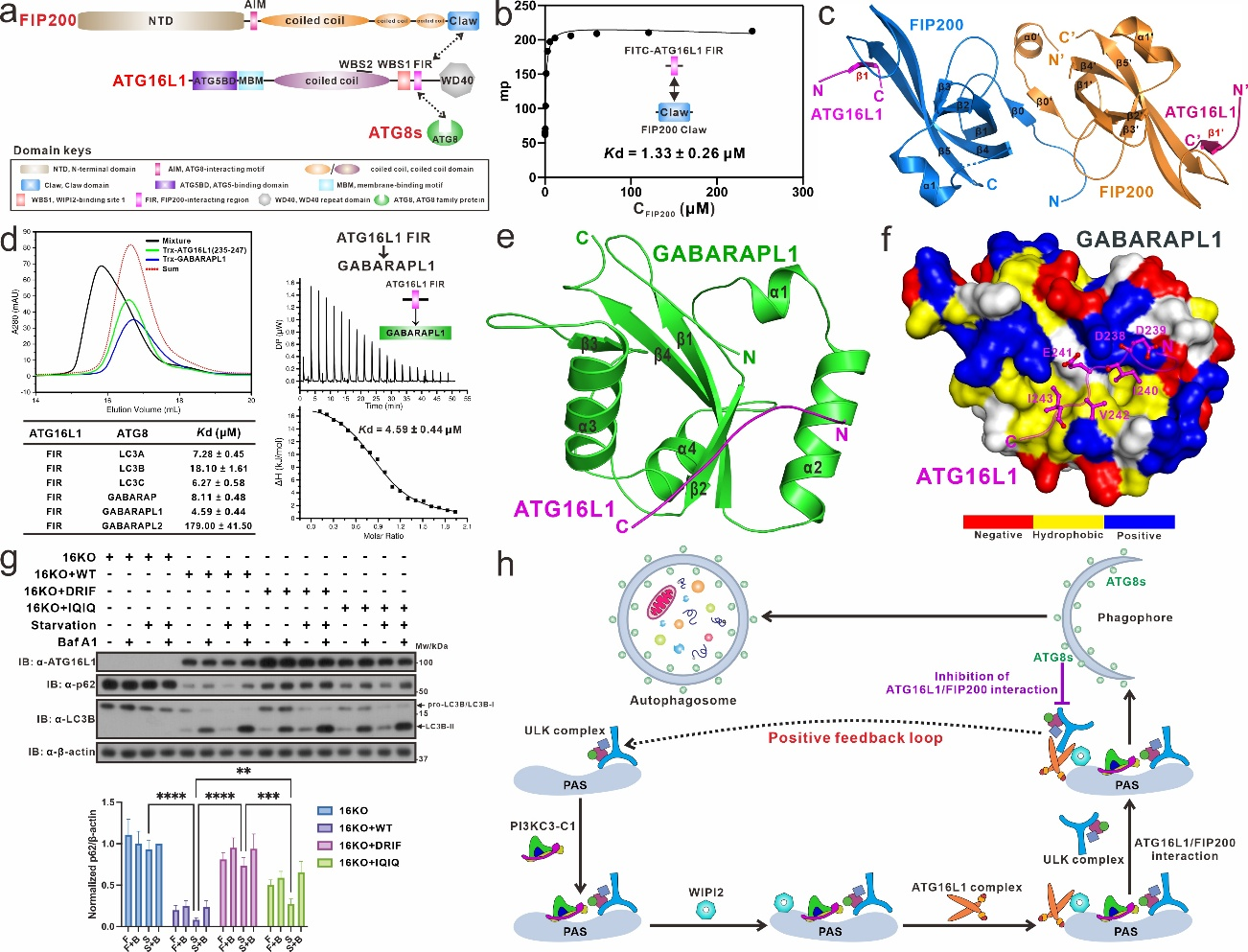
Figure 1. Mechanistic insights into the interaction of ATG16L1 with FIP200 and ATG8 family proteins
PAN Lifeng, Ph.D. Professor Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences Ling Ling Road 345 Shanghai 200032 China Tel: 0086-21-54925561 Email: panlf@sioc.ac.cn |


